| WELLPATH COLLABORATES WITH HI-BRIDGE HIE AND MOREHOUSE SCHOOL OF MEDICINE TO PROVIDE IMPROVED CARE DELIVERY FOR GEORGIA CORRECTIONAL PATIENTS NASHVILLE, Tennessee – Wellpath recently announced an alliance with the Morehouse School of Medicine and the HI-BRIDGE Health Information Exchange (HIE) to electronically share real-time correctional patient health information for care delivery throughout 70 facilities in Georgia. This alliance enables the exchange of real-time, secure healthcare data between the HIE and Wellpath’s electronic health record platform, Electronic Records Management Application (ERMA). The data can include medication history, prior diagnoses, allergies, and lab reports from any one of the many contributing sources. The bi-directional data flow between Wellpath and HI-BRIDGE HIE also enables clinical services provided by Wellpath to be added to the patient record and shared with other local and national healthcare providers and organizations when the patient seeks medical care following release. “Communication is crucial as it relates to healthcare delivery in our facilities and incorporating this model will help ensure information is being shared efficiently and in real-time,” said Georgia Department of Corrections Commissioner Timothy C. Ward. “We appreciate our partners at Wellpath and their commitment to continuous process improvement involving healthcare for our offender population.” The HI-BRIDGE HIE is an independent regional information exchange established through the National Center for Primary Care at Morehouse School of Medicine and connected to the Georgia Health Information Network (GaHIN). The simplified exchange of comprehensive, real-time patient health information supports care coordination and optimal health outcomes by eliminating disparities and improving access to health information. The Wellpath/HI-BRIDGE HIE relationship goes back to 2017, connecting a network of local county jails with the exchange of patient health information. “This is a unique model of data exchange partners covering public, private, and academic experts,” said Carmen L. Hughes, MBA, executive director, HI-BRIDGE HIE. “Through the partnership, Wellpath clinicians will have patient clinical records at the point of care or in a healthcare crisis; this is critical for continuity of patient care,” Hughes said. Wellpath continuously seeks ways to improve the quality of healthcare delivered to its patients. “Health Information Exchanges are an important component in the delivery of quality healthcare, and we believe our patients are best served when we provide them with the most informed care possible,” said Jorge Dominicis, CEO of Wellpath. “When one of our healthcare professionals meets a patient for the first time and immediately has access to the patient’s entire medical history, that patient can receive optimal care, and that is always our goal.” Wellpath expanded its relationship with Morehouse School of Medicine in November 2021 by helping to offer an Online Education and Expanded Programs (OEEP) certificate that promotes health equity for justice-involved individuals. Wellpath clinicians are helping build another course on the impact of social determinants of health (SDoH) in correctional settings, with the goal to encourage community resources that eliminate barriers to care for justice-served patients. “This special relationship in education between Wellpath and Morehouse began in November 2021 when the two organizations recognized the need for medical programs focused on incarcerated patients,” explained Dominic H. Mack, MD, MBA, executive medical director, HI-BRIDGE HIE, director, National Center for Primary Care, and faculty member for Family Medicine, at the Morehouse School of Medicine. “Together, we designed a self-directed online certificate for coaches wanting to help justice-involved individuals. Technology plays a significant role in primary care practice,” said Dr. Mack. Students with a bachelor’s degree are guaranteed admission into Morehouse School of Medicine, pending SACSCOC approval of a Master of Administration in Justice-Involved Care (MAJIC) degree program. They also receive a scholarship, he said. ### ABOUT WELLPATHWellpath is the premier provider of localized, high-quality, compassionate care to vulnerable patients in challenging clinical environments. Wellpath holds patients at the center of everything we do and promotes rigorous standards of care and innovation. With over 14,200 clinicians and professionals in 36 states across the U.S. and Australia, Wellpath provides medical and mental healthcare services for over 240,000 patients daily in more than 550 facilities, including prisons, jails, state hospitals, forensic treatment, civil commitment centers, and community care centers. Learn more at www.wellpathcare.com. ABOUT MOREHOUSE SCHOOL OF MEDICINEFounded in 1975, Morehouse School of Medicine (MSM) is among the nation’s leading educators of primary care physicians, biomedical scientists, and public health professionals. An independent and private historically Black medical school, MSM was recognized by the Annals of Internal Medicine as the nation’s number one medical school in fulfilling the creation and advancement of health equity. MSM faculty and alums are known for excellence in teaching, research, public policy, and exceptional patient care. The Commission accredits MSM on Colleges of the Southern Association of Colleges and Schools to award doctoral and master’s degrees. To learn more about programs and donate today, please visit www.msm.edu or call 404-752-1500. ABOUT THE HI-BRIDGE HEALTH INFORMATION EXCHANGE (HIE)HI-BRIDGE HIE is an independent regional exchange established through the National Center for Primary Care at Morehouse School of Medicine and connected to the Georgia Health Information Network (GaHIN). The regional exchange, based in Atlanta, provides interoperability through integrated technology and clinical support service to meet the needs of smaller practices, health systems, and other providers of healthcare delivery for the electronic exchange of patient clinical information. 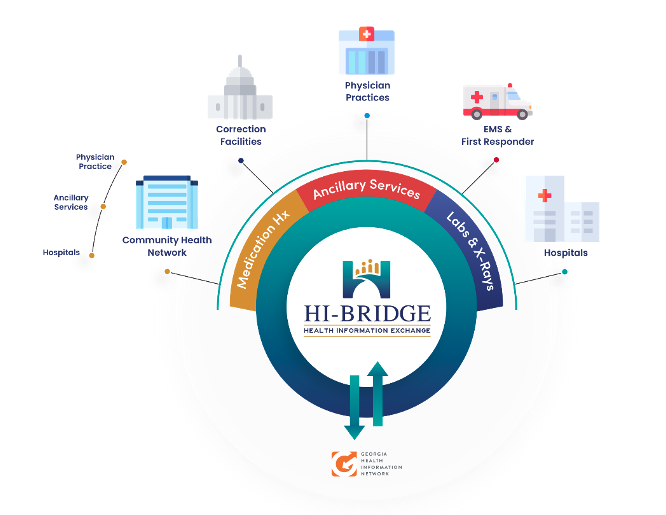 Wellpath / 3340 Perimeter Hill Drive / Nashville, TN 37211 www.wellpathcare.com Wellpath / 3340 Perimeter Hill Drive / Nashville, TN 37211 www.wellpathcare.com |
Author: trainitright
Alimentiv, Satisfai Health, and Virgo Announce Partnership to use AI-driven technology to Enhance Clinical Trials in IBD and other GI Diseases
The partnership enables advanced AI-fueled HD video capture, decision support, and clinical research tools for Alimentiv's global clinical trial investigator site network.
LONDON, ON, Oct. 5, 2022 /CNW/ - Alimentiv Inc. ("Alimentiv"), Satisfai Health, Inc ("Satisfai"), and Virgo Surgical Video Solutions, Inc. ("Virgo") today announced a strategic partnership to revolutionize central reading, data capture, decision support, and patient recruitment in the clinical trial domain for Inflammatory Bowel Disease (IBD) and allied GI conditions. Satisfai, a global leader in developing artificial intelligence solutions for gastroenterology, and Virgo, an industry leader in endoscopy video capture technology, will leverage their combined strengths to work in partnership with Alimentiv, a leading CRO in gastroenterology, to realize the full potential of AI-powered precision imaging in the GI clinical trials space.

"This synergy of Satisfai and Virgo with Alimentiv is a positive development for patients with IBD and the drug development space. Clinical trials have many pain points, and this strategic partnership will enable advances in improving the efficiency of clinical trials and make some significant changes to how trials are run", said Jeff Smith, CEO of Alimentiv. "We are delighted to enter this arrangement to bring the next generation of imaging technology to clinical trials in gastroenterology."
"Artificial Intelligence in medicine is being increasingly adopted across various clinical specialties, with gastroenterology being one key field of use, particularly endoscopy," said Dr. Michael Byrne, CEO and founder of Satisfai Health, Clinical Professor of Medicine and gastroenterologist in Vancouver. "Working with Virgo, we are excited to bring AI-driven solutions to the IBD clinical trials arena. To be able to do so with Alimentiv — a leading Gastroenterology and imaging CRO—is a great opportunity. Our clear ambition is to change the clinical trial paradigm for IBD and other disease states such as Eosinophilic Esophagitis. As three groups working together, we are confident we can do so."
"Combining Satisfai's marquis AI solutions with Virgo's HIPAA and SOC 2 compliant automated cloud video capture technology and Alimentiv's 21CFR11 compliant solutions, expansive site community, and imaging expertise is very powerful. We truly look forward to realizing this vision and bringing real-time, in-line AI solutions to GI clinical trials worldwide," said Matthew Schwartz, CEO and co-founder at Virgo.
This partnership will facilitate Alimentiv's continued leadership in GI Clinical Trials through exclusive access to Satisfai's SmartScore Central Reading tool and Virgo's fully integrated, hands-free, cloud-based high-definition video-capture fleet. In addition, this will provide pharmaceutical and biotech sponsors with access to the ongoing development of novel endpoints, scoring methodologies, and scalable solutions geared toward bringing novel therapies to market faster.
About Alimentiv Inc.
Alimentiv is a global contract research organization (CRO) providing clinical trials, central image management, precision medicine, and real-world evidence services to the pharmaceutical and biotechnology industries. Headquartered in London, Ontario, Alimentiv employs more than 500 people across its operations in Canada, the United States, Europe, Asia-Pacific, and Latin America. The organization's unique model combines the efforts of internationally recognized academic researchers and operational experts to offer integrated solutions to customers. Over the past 20 years, Alimentiv has become a recognized expert in clinical trial design, central image management solutions, outcome measure development, and precision medicine for drug development in IBD. Today, Alimentiv provides services in more than 50 countries worldwide, collaborates with leading universities and academic institutions across the globe, and partners with many leading pharmaceutical and biotechnology organizations to bring new and improved treatment options to patients. Alimentiv is committed to investment in medical research and development, focusing on identifying barriers to drug development and pursuing solutions that advance IBD research. The research findings are operationalized into an efficient clinical trial methodology for clients that aligns with emerging regulatory standards. In collaboration with leading experts, Alimentiv has pioneered the development, validation, and standardization of outcome measures and technology, shaping the evolving clinical trial landscape for multiple indications and providing meaningful long-term consequences for patients, their treatment, and society. For more information, visit: www.alimentiv.com
About Satisfai Health
Satisfai is a leading medical solutions provider specializing in AI applications applied to large addressable markets in gastroenterology. Satisfai's solutions deliver real-time medical imagery analysis, providing clinicians with decision support intelligence that dramatically improves patient outcomes. Satisfai is supported by a highly respected board of medical clinicians and key opinion leaders who operate at the top of their fields in the many areas of gastroenterology. Satisfai enjoys a strong voice on academic panels, leading GI societies, and direct access to prominent industry players seeking to adopt new AI technologies in gastroenterology. For more information, visit www.satisfai.health or email andrew@satisfai.health
About Virgo
Virgo provides the leading cloud video capture, management, and artificial intelligence analysis platform for endoscopic medicine. Academic, integrated, and private practice healthcare providers use the Virgo platform to advance patient care through video-based research and training initiatives. Since launching, Virgo has helped physicians capture over 400,000 endoscopy procedures using industry-leading HIPAA, HITRUST, and SOC 2-compliant cloud service providers. Virgo also supports integration with all leading electronic health records systems. In 2021, Virgo launched a suite of tools called VirgoTrials, which help pharmaceutical trial sponsors and their participating trial sites accelerate patient recruitment and shorten the overall enrollment period for trials. For more information, visit www.virgosvs.com or email jake@virgosvs.com
SOURCE Alimentiv Inc.
PharmaTher Holdings Announces Grant of U.S. Patent Covering Ketamine for Parkinson’s Disease
Grant of US patent strengthens the PharmaTher’s intellectual property portfolio covering novel uses and delivery forms of ketamine
Announced positive efficacy and safety data from Phase 1/2 clinical study of ketamine in the treatment of levodopa-induced dyskinesia in Parkinson’s disease patients, with 100% of patients treated with ketamine demonstrating a reduction in dyskinesias as measured by the Unified Dyskinesia Rating Scale showing a 51% reduction from baseline during Infusion 2 (p=0.003), 49% at 3-week (p=0.006) and 41% at 3-month (p=0.011) post-ketamine
Planning a Phase 3 clinical study to allow for FDA approval of KETARX™ (ketamine) for Parkinson’s disease under the 505(b)(2) regulatory pathway
TORONTO, Oct. 05, 2022 (GLOBE NEWSWIRE) -- PharmaTher Holdings Ltd. (the “Company” or “PharmaTher”) (OTCQB: PHRRF) (CSE: PHRM), a specialty pharmaceutical company focused on developing and commercializing novel uses and delivery forms of ketamine to treat mental health, neurological and pain disorders, is pleased to announce that the United States Patent and Trademark Office (“USPTO”) granted US Patent No: 11,426,366, titled “Compositions and Methods for Treating Motor Disorders,” which includes claims intended to cover ketamine in the potential treatment of Parkinson’s Disease and motor disorders that cause involuntary or uncontrollable movement or actions of the body (the “Patent”).
Fabio Chianelli, CEO of PharmaTher, commented: “We are pleased with the USPTO grant of the patent covering ketamine in the treatment of Parkinson’s disease and motor disorders. The US patent strengthens our intellectual property portfolio covering novel uses and delivery forms of ketamine. We are advancing the clinical development of ketamine in the treatment of levodopa-induced dyskinesia in patients with Parkinson’s disease, and we are seeking FDA agreement on pursuing approval under the 505(b)(2) regulatory pathway with a potential Phase 3 clinical study.”
On September 16, 2022, the Company announced positive efficacy and safety data from a Phase 1/2 clinical study of ketamine in the treatment of levodopa-induced dyskinesia (“LID”) in Parkinson’s disease via a late-breaking abstract presentation, titled “Subanesthetic infusion of ketamine produces long-term reduction in levodopa-induced dyskinesia” at the MDS International Congress of Parkinson's Disease and Movement Disorders®.
The Phase 1/2 study was an open-label, dose-finding trial to test the safety, tolerability and pharmacokinetics of low-dose ketamine infusion to treat levodopa-induced dyskinesia in Parkinson’s disease and to find an effective dose-range suitable for outpatient use. Two 5-hour low-dose ketamine infusions were given within a one-week period. The clinical study outcomes measured reduction of dyskinesia, captured with the Unified Dyskinesia Rating Scale (“UDysRS"), and effects on parkinsonian symptoms, captured with the Unified Parkinson's Disease Rating Scale (“UPDRS”).
The study enrolled 10 subjects with moderate to advanced Parkinson’s disease with a target infusion rate being 0.30 mg/kg/hr. These data highlight that ketamine was safe, well-tolerated, and demonstrated that 100% of subjects treated with ketamine had a reduction in dyskinesias as measured by UDysRS indicating possible efficacy with a large effect size that warrants further investigation in a pivotal Phase 3 clinical study. UDysRS showed a clinically meaningful reduction from baseline through 3 months.
| Infusion 2 | 3-weeks post Infusion | 3-months post Infusion | |
| UDysRS Percent Reduction | 51% (p=0.003) | 49% (p=0.006) | 41% (p=0.011) |
| UPDRS Percent Reduction | 27% (p=0.057) | 28% (p=0.026) | 5% (p=0.258) |
The maximum tolerated infusion rate ranged from 0.20-0.30 mg/kg/hr which was dependent on either discomfort due to dissociation or hypertension. There were no adverse events post-infusion. Analyses of the patient diaries and the pharmacokinetic data are ongoing.
Based on these data, the Company is preparing to engage the FDA to establish the next steps for a planned Phase 3 clinical study to allow for the Company’s proprietary ketamine intravenous product, KETARX™, approval for Parkinson’s disease under the 505(b)(2) regulatory pathway. In August 2020, the Company entered into an exclusive license agreement with the University of Arizona to develop and commercialize the Patent.
Ketamine’s Potential In Parkinson’s Disease
Parkinson’s disease is a debilitating disorder that affects an estimated 1 million people in the U.S. and 10 million people worldwide. The global Parkinson’s disease market is expected to grow from USD $5 billion in 2019 to USD $7.5 billion by the end of 2025 [360iResearch 2020] and it is estimated that the potential market opportunity for LID Parkinson’s disease to be over USD $3 billion in the U.S. alone.
Ketamine is an FDA-approved N-methyl-D-aspartate receptor-modulating (NDMA) drug that is widely used as an anesthetic agent either alone or in combination with other anesthetic agents [Smith et al, 1987; Pacheco et al, 2014]. The possible therapeutic effect of low-dose ketamine on levodopa-induced dyskinesia was noted in a retrospective analysis of Parkinson’s disease patients who received ketamine for pain relief. During this analysis, it was observed that the patients experienced an improvement in LID lasting several weeks beyond treatment [Sherman et al, 2016]. These results were corroborated in a test of low-dose ketamine in a rodent LID model, and this possible effect has also been examined in a controlled study [Bartlett et al, 2016]. Ketamine may also have additional benefits in the treatment of pain [Niesters et al, 2014] and depression [Diamond et al, 2014; Murrough et al, 2013], which are frequent comorbidities of Parkinson’s disease.
About Parkinson’s Disease
There is currently no cure for Parkinson’s disease, although some drug combinations are used to treat the disease symptoms. Although the etiology of Parkinson’s disease is not fully understood, it is thought to result from loss of pigmented dopaminergic neurons in the Substantia nigra and their striatal projections, leading to dopamine deficiency in the striatum [Schapira and Jenner, 2011]. This ultimately affects the cortico-striatal system that controls movement. As a progressive neurogenerative disorder of the central nervous system that primarily affects the motor nerve system, symptoms of Parkinson’s disease may emerge slowly and include tremors, rigidity, bradykinesia, and postural instability [Paulson and Stern, 2004]. Also, patients may experience non-motor symptoms such as autonomic dysfunction (orthostatic hypotension, constipation, bladder dysfunction), psychiatric (depression), cognitive and sensory symptoms (pain) [Olanow, et al, 2009]. These non-motor symptoms become more common as the disease progresses. Treatments, including levodopa and dopamine agonists, which restore the dopamine deficits in the brain, have been employed for almost 50 years. However, with continued treatment using levodopa, dose-limiting motor side-effects often emerge. This includes the emergence of abnormal involuntary movements termed Levodopa Induced Dyskinesias, which can be identified in about 50% of patients within five years after initiation of levodopa treatment and in almost all patients within ten years post-treatment initiation. These side effects often limit further dose increases in dopaminergic therapy.
There can be no assurance that the FDA will support any potential request for an expedited path to approval or further development for ketamine in the treatment of Parkinson’s disease.
About PharmaTher Holdings Ltd.
PharmaTher Holdings Ltd. (OTCQB: PHRRF) (CSE: PHRM) is a specialty pharmaceutical company focused on developing and commercializing novel uses and delivery forms of ketamine to treat mental health, neurological and pain disorders. PharmaTher’s product portfolio consists of KETARX™ (ketamine) delivered by intravenous injection, intradermal microneedle patch, and subcutaneous pump administration. Learn more at PharmaTher.com.
Expert Shares Essential Traits of Strategic, Effective Leadership
Dallas, TX, October 5, 2022 — Some people actively pursue leadership positions, while others have the responsibility thrust upon them. Whatever the case, effective leadership is always in short supply, even though many people hold positions that require it, according to consultant Mark Rapier.
“Your personal leadership journey will not be a straight line to success,” Rapier said. “You will need to ebb and flow when you find yourself in those leadership moments, being prepared with expanded views.”
His new book, The Leader With a Thousand Faces: A Personal Study of Leadership,
describes the journey that leaders must learn how to navigate and aims to give readers perspectives to consider before they find themselves needing answers. Written from Rapier’s experience, not theory, readers will find the clues to uncovering the best versions of the leaders they choose to be.
Rapier explores leadership types (including some interesting combos), dispels myths and then gets into the nitty gritty of day-to-day leadership duties. Readers will also find situational examples, self-evaluation tools and answers to common questions, such as:
- What is the difference between good leaders and those who just think they are?
- What is the difference between management and leadership?
- Why do some techniques used successfully in the past no longer work?
- How can those in leadership roles demonstrate that they are ready for more leadership responsibilities?
- And much more.
“Leadership is in all of us,” Rapier added. “All we need to do is unlock our potential.”
About the Author
Over more than 40 years, Mark Rapier has acquired skills and experiences across multiple industries and platforms, which has given him valuable perspectives. As an adviser, Rapier works with C-suites, IT leadership and business stakeholders to develop and execute effective digital transformation strategies and IT operating models. He has experience with the automotive, financial services, insurance, life sciences, logistics, retail, utilities and U.S. public sectors, and has worked with global organizations with revenues ranging from $300M to $160B.
He has worked with hundreds of leaders from all over the world and led global organizations with more than 1,500 team members. He drew upon this experience and extensive research to inform The Leader With A Thousand Faces.
He holds a Bachelor of Business Administration degree from The University of Texas at Austin. He lives in Arlington, Texas, and is an avid and below-average golfer. For more information, visit https://www.rapiergroupllc.com/, or follow the author on https://medium.com/the-leaders-journey or https://www.linkedin.com/in/markrapier/.
The Leader With a Thousand Faces: A Personal Study of Leadership
Publisher: Bowker
ISBN-13: 979-8986059310 (paperback)
ISBN-13: 979-8986059303 (e-book)
Available from Amazon.com
###
Newborn’s First 1,000 Days Crucial to Preventing Diseases, Allergies — New Book
Philadelphia, October 5, 2022 — Expectant moms are advised to eat well, exercise, take vitamins, abstain from caffeine and do everything possible to ensure the optimal health of their babies. Yet, children born today are sicker than in any past generation. Food allergies, asthma and other chronic health conditions affect up to 30 percent of kids across the country. Why?
In The Baby and the Biome: How the Tiny World Inside Your Child Holds the Secret to Their Health (Avery, an imprint of Penguin/Random House; September 6, 2022; Hardcover; ISBN: 9780593421024), author Meenal Lele reveals the answer—and it’s not bad genes or bad parenting. As a medical researcher with a chemical engineer’s degree from Wharton, her firsthand experience as an “allergy mom,” Lele sheds light on the key role of a newborn’s microbiome (the mix of bacteria, fungi, and viruses that live on us and in us) in either preventing or provoking diseases of the immune system, which include not only food allergies and asthma but also eczema, ADHD, IBS, type 2 diabetes, Crohn’s disease and more.
“Immune disease needs a revolution in thinking—and an accessible way for parents to understand it,” Lele asserts. Interweaving the story of her firstborn son Leo’s life-threatening struggles with food allergies and asthma with significant, potentially life-saving scientific findings, Lele aims to lead that revolution by educating parents about how to prioritize and strengthen their baby’s microbiome by protecting the delicate skin, gut and lung barriers.
In accessible language and a reassuring tone, The Baby and the Biome combines medical insights with practical advice on a more informed, healthier approach to the ABCDEs—Antibiotic use, Baby Care, Diet and Environment—during a newborn’s first thousand days, and helps parents raise kids in this toxin-filled world. You’ll learn:
- How to nurture your baby’s biome during pregnancy, practice good biome care from the moment of birth and, if possible, choose breastfeeding over formula to support immunity—but only exclusively for the first two months of your baby’s life.
- Why many standard practices endorsed by pediatricians—wash your baby daily, wait three days between introducing your baby to new foods and more—are not backed by science and might actively hurt your child’s immune defenses.
- Why eczema, which affects about 25 percent of infants, is not just “a little rash” but both a serious warning sign and direct cause of increasingly worse immune conditions to come—plus, clinically proven, actionable ways to treat it.
- Why antibiotics commonly prescribed for ear infections and fever are often unnecessary and can actually make a child sick longer and cause diarrhea, among other complications—plus, examples of when antibiotics are essential.
- Why to avoid using creams and lotions, including sunscreen, on your baby’s skin—and tips for minimizing diaper rash, including using cloth diapers (which are also better for the environment than super-absorbent disposable diapers).
- Why giving your baby regular exposure to the most common food allergens, including peanuts and eggs, is a vital practice for developing a healthy, disease-free immune system—and how to do it safely and stress-free.
- The importance of feeding your baby a good and diverse diet, with priority on avoiding added sugar and processed foods, which contain preservatives, dyes, synthetic fats and emulsifying agents that are poisonous to the gut barrier.
- The biome-friendly benefits of letting your baby play and crawl around in good dirt, whether in your own backyard or a natural park with real grass, trees and mud, as opposed to a playground built on artificial turf.
- Tips for cleaning your home, dishes and clothes, as well as your family’s hair and bodies, to prevent immune disorders—plus, the potential long-term dangers of our national obsession with antibacterial cleansers … and much more.
About the Author
Meenal Lele is a mom to two boys, medical researcher and chemical engineer with a degree from the Wharton School. She is the founder and CEO of Lil Mixins, a company devoted to educating parents about how to prevent their children from developing food allergies. She lives in Philadelphia, PA, with her family.
From Homeless to the C-Suite: CEO Traces Improbable Journey in The Most Unlikely Leader
West Palm Beach, FL, October 5, 2022 — At age 15, Roger Smith dropped out of school, left home and became addicted to drugs. By all accounts, he should have hit rock bottom multiple times before that fateful day, nearly 20 years later, when he found himself on his hands and knees, rummaging for remnants of crack cocaine in the carpet. It’s an image of himself he says he never wants to recreate.
“The picture of who I was at the very end — that picture was so strong in my mind that I just never wanted to go back there again,” Smith said during a recent interview.
The Most Unlikely Leader: An Unbelievable Journey From GED to CEO is Smith’s empowering story of how he got clean, got his act together and rose through the ranks of American Income Life Insurance, where he eventually became CEO. But Smith didn’t stop there. He also went on to serve as CEO of National Income Life Insurance and Liberty National Life Insurance companies.
A fast-paced blend of memoir, business manifesto and leadership manual, The Most Unlikely Leader is an unflinching look at a side of Smith’s life that few people knew about — until now. He takes readers inside his philosophy, his decisions (both good and bad) and the sheer grit he harnessed to overcome seemingly insurmountable obstacles.
Smith speaks candidly about his struggles with addiction, divorce, lack of formal education and homelessness in an effort to show readers that problems can be solved, bad habits can be broken, and challenges can be overcome with the right mindset and work ethic.
“I want to share my story to tell you that your life and your career and who you are as a person are all within your hands,” he wrote in the book’s introduction. “You can fall all the way down to a dark place where you can’t see your way out … and then through sheer grit and gumption and tenacity … you can claw your way out. Because that’s what I did.”
About the Author
Roger Smith is an American author and former CEO of American Income Life Insurance, National Income Life Insurance and Liberty National Life Insurance companies. His journey is one that embodies the fact that no matter how low a person is in life, almost anything is possible with determination and the right mindset. He was the recipient of the Yitzhak Rabin Legacy Award, Eleanor Roosevelt Human Rights Award, Healthcare For All Champion Award, Sol Stein Award and numerous others. He is the father of five adult children (Nicole, Conrad, Emily, Adam and Amiah), proud grandfather of Maggie Mae, and currently resides in Florida with his wife, Demi, and his two dogs, Penelope and Chrome.
For more information, visit https://rogersmith.me/, or follow the author on Instagram (rogersmithceo), TikTok (rogersmithceo) or Facebook (@rogersmithceo). Watch Smith read his book’s introduction here: https://m.facebook.com/rogersmithceo/videos/the-most-unlikely-leader-introduction-read-by-roger-smithy/424545199293561/.
The Most Unlikely Leader: An Unbelievable Journey From GED To CEO
Publisher: Ballast Books
ISBN-10: 1955026130
ISBN-13: 978-1955026130
Available from Amazon.com, BN.com, Target.com and https://rogersmith.me/
Framework for Diabetes in Canada tabled in Parliament
TORONTO, Oct. 05, 2022 (GLOBE NEWSWIRE) -- Today’s tabling of the Framework for Diabetes in Canada by the Honourable Jean-Yves Duclos, federal Minister of Health, in the House of Commons, is an exciting and welcome development that aims to address the complex condition and rising prevalence of diabetes in Canada. Diabetes Canada expresses thanks to the Minister, MP Sonia Sidhu, Chair of the all-party Diabetes Caucus, and her parliamentary colleagues, whose commitment and leadership helped make this tabling a reality.
For the past several years, Diabetes Canada has led the call for a strategy and framework, in collaboration with more than 100 groups and individuals from across the country, to address the growing and silent epidemic of diabetes which impacts more than 11.7 million Canadians and costs the health-care system almost $50 million to treat every day.
Diabetes Canada is pleased with the measures outlined in the much-anticipated Framework document and urges the federal government, and its provincial and territorial partners, to identify and dedicate the necessary investments in their 2023 Budgets (and successive years) to implement the Framework.
These investments should include improved access to treatments, risk reduction strategies, and enhanced research all with one ultimate objective—to improve health outcomes for people with diabetes. Diabetes Canada recommends that a convening and coordinating body that includes people living with diabetes, national experts in care and research, and public and private funding partners should be established to help drive the implementation and evolution of this Framework and report back on progress on an annual basis.
The Framework was developed in consultation with people impacted by diabetes, health groups, and all levels of government, and has the potential to prevent millions of diabetes diagnoses as well as ensure that all those living with diabetes have improved and equitable access to vital care.
More specifically, federal, provincial and territorial governments must invest in its implementation to support the following:
- Access to Resources: make sure people who live with diabetes have timely and equitable access to the supports, medications, and devices they need.
- Measurable Progress: create and fund a multisectoral coordinating body to drive and ensure accountability so effective actions are taken, progress is tracked, and best practices are used.
- Comprehensive Data: scale up and expand current data sources, and increase data sharing, through new data connection points that will improve health outcomes.
- Better Education: improve public understanding about diabetes as a disease with tools that are inclusive, focused on the individual, and reduce stigma and inequities.
- More Research: renew innovation with comprehensive research into type 1 and type 2 diabetes.
Quotes:
Laura Syron, President & CEO, Diabetes Canada and person living with type 2 diabetes
“We applaud the government for moving forward with a plan of action to support Canadians at risk of and living with diabetes. The release of today’s Framework for Diabetes in Canada provides a critical and foundational outline that will guide us in helping to improve access to care and treatment and ensuring better health outcomes for people in Canada. We must keep the momentum moving and look forward to collaborating with governments, health-care partners, and persons with diabetes to ensure the Framework is implemented through concrete investments as soon as possible.”
Stacey Livitski, person living with type 1 diabetes
“I am so thankful that this comprehensive plan is gaining traction to help address the growing impact of the diabetes crisis across our nation. I am hopeful that as a result people like me will be able to see and benefit from some long overdue changes that will provide us with more measurable and positive outcomes, helping to alleviate the burden on individuals as well as our already overburdened health-care systems. I am urging the government to invest and implement these strategies as soon as possible.”
Russell Williams, Senior Vice-President of Mission, Diabetes Canada
“As the Framework for Diabetes in Canada is implemented across the country, there will be direct applications and learnings for other chronic diseases, facilitating economies of scale, rapid knowledge-sharing and bringing transformative change that will be felt throughout the health-care system.”
About Diabetes Canada
A world free of the effects of diabetes is our vision. That’s why we’re working together to improve the quality of life of people living with diabetes. We’re sharing knowledge and creating connections for individuals and the health-care professionals who care for them; advocating through public policy; and funding research to improve treatments and find a cure to end diabetes.
For more information, visit diabetes.ca or call 1-800-BANTING (226-8464).
Largest study measuring extent of COVID-19 in Canadian children and teens now underway
MONTREAL, Oct. 06, 2022 (GLOBE NEWSWIRE) -- In Canada, the vast majority of COVID-19 cases in the 0 to 18 age group have been mild or asymptomatic. This fact, combined with the reduction in routine COVID-19 laboratory testing across most of the country, means that the infection rates in children and adolescents are largely unknown. What’s more, since levels of transmission, vaccination and immunity are continually changing, ongoing surveillance is necessary to help guide public health policy.
To address the knowledge gap, the Government of Canada and the Canadian Institutes of Health Research (CIHR), through the COVID-19 Immunity Task Force (CITF), are providing $2.6 million to conduct the largest serosurvey of children and youth to date in Canada for SARS-CoV-2. The study, which benefits from the network established for an existing CIHR-funded project called Pediatric Outcomes Improvement through Coordination of Research Networks (POPCORN), is called COVID-19 seroepidemiology among children Using Retrieved POPCORN Site Leftover blood Samples (CURNLS). It is led by Drs. Soren Gantt and Caroline Quach-Thanh, investigators at CHU Sainte-Justine Research Centre and professors at the Université de Montréal. POPCORN is also led by Dr. Quach-Thanh and brings together pediatric health researchers from 16 hospital research sites across Canada to monitor COVID-19 infections, vaccination, and social impacts among children and youth.
“The CURNLS study involves testing existing blood samples from patients aged 0 to 18 who visit hospital emergency departments across Canada with the goal of identifying whether they’ve had COVID-19 and whether they have immunity from infection or vaccination,” says Dr. Gantt. “We will combine this information with rates of transmission, hospitalization, vaccination, and use of public health measures to inform public health policy.”
“By including large numbers of children from the POPCORN network sites, which span eight provinces, the CURNLS study will provide relatively broad and representative seroprevalence data among children and youth across Canada,” says Dr. Quach-Thanh. By testing for antibodies to SARS-CoV-2, including nucleocapsid (N), spike (S) and receptor-binding domain (RBD) antigens, the CURNLS researchers have several goals. “We aim to determine the rates of seropositivity due to infection and vaccination, the differences in seroprevalence among children of different ages and from different regions of Canada, and the associations between serologic measures and trends of viral transmission and vaccination rates,” says Dr. Quach-Thanh.
Five times during the coming year, approximately 7,200 samples will be obtained and tested through the new study. Analyses will be performed according to three distinct age groups within the 0 to 18 range.
“The CITF funds several studies, including among blood donors via Canadian Blood Services and Héma-Québec, that provide valuable seroprevalence data for adults on an ongoing basis, but until now there has been no nationally representative and ongoing pediatric population included,” says Dr. Tim Evans, CITF Executive Director. “The CURNLS study is the first national serosurvey focussing on children and teens, and the data it generates on SARS-CoV-2 infection and immunity will help predict future pandemic trends, understand the spectrum of disease arising from infection in this age group, the role of children in community transmission, and the need for additional vaccination and/or public health measures.”
About the COVID-19 Immunity Task Force
The Government of Canada established the COVID-19 Immunity Task Force (CITF) in late April 2020 to catalyze, support, fund, and harmonize research on SARS-CoV-2 immunity for federal, provincial, and territorial decision makers in their efforts to protect Canadians and minimize the impact of COVID-19. The Task Force and its Secretariat work closely with a range of partners, including governments, public health agencies, institutions, health organizations, research teams, other task forces, engaging communities and stakeholders. To date, the CITF has supported over 110 studies across Canada that are generating critical insights on the levels, trends, nature, and duration of immunity arising from SARS-CoV-2 infection and COVID-19 vaccination. The CITF is overseen by an Executive Committee of volunteers that includes leading scientists and policymakers from across Canada.
About Canadian Institutes of Health Research
At the Canadian Institutes of Health Research (CIHR), we know that research has the power to change lives. As Canada’s health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
YBVR, TELUS, and MEDIAPRO Canada join forces to deliver a cutting-edge immersive viewing experience for Canadian Premier League soccer fans
Canadian soccer fans can now watch Canadian Premier League for free on the TELUS Sports app available on mobile devices through the Apple App Store and Google Play Store
VANCOUVER, British Columbia, Oct. 06, 2022 (GLOBE NEWSWIRE) -- YBVR, a Silicon Valley technology startup and a global leader in Immersive Live Sports streaming, has joined forces with TELUS and MEDIAPRO Canada to pilot a fully-immersive Sports Metaverse service in Canada and redefine the way fans experience live sports. Beginning with OneSoccer and the Canadian Premier League (CPL), the TELUS Sports app brings Canadian soccer fans access to unique features allowing them to choose, in real time, between up to eight different camera angles, across multiple devices and from multiple viewers to offer the most immersive soccer viewing experience in the comfort of their home or on-the-go. TELUS Sports is free and available for download on mobile devices through the Apple App Store and Google Play Store, select Android TVs for TELUS subscribers on Optik TV and Pik TV, and web browsers by visiting telussports.ybvr.com.
“YBVR is proud to partner with TELUS and MEDIAPRO Canada to offer a one-of-a-kind viewing experience to Canadian soccer fans,” said Hector Prieto, Co-Founder and CEO at YBVR. “YBVR is the technology provider behind the enhanced, immersive, multi-camera and hyper-personalized fan experience. It is an interactive and flexible solution that will be available for all October Canadian Premier League games. As soccer fever takes over Canada, the ending of this year’s season promises to be exciting, and TELUS, combined with the power of YBVR’s technology, will make it accessible for all Canadians.”
YBVR’s current portfolio of immersive sports tech initiatives includes projects with Wimbledon and the Australia Open tennis Majors, EuroLeague basketball, and Real Madrid, as well as partnerships with major players in sports broadcasting such as Verizon, Singtel, and Telefónica.
“As the sport with the highest participation levels among children and young people in Canada, soccer is embedded in our national mainstream. This project provides an exciting boost for fans by enhancing the Canadian Premier League’s digital content offering through the TELUS Sports app,” said Amit Nag, Vice-president, Entertainment and Education Services at TELUS. “By partnering together with YBVR and MEDIAPRO Canada, TELUS is piloting next-generation technology for the Canadian Premier League and enhancing soccer’s impact on the Canadian landscape.”
The new partnership is among the first to ride the wave of technological innovation into Canada, bringing immersive, personalized and interactive functionalities to soccer. The combined technological capabilities and experience of these three world-leading companies will bring a trailblazing new option to Canadian Premier League fans through the TELUS Sports app and OneSoccer.
“This new venture represents an extraordinary opportunity for OneSoccer and the Canadian Premier League and will vault the viewing experience to a whole new level,” said Oscar López, Chief Executive Officer at MEDIAPRO Canada. “In just four years, the CPL has developed into a compelling and competitive league with a dedicated fan base from coast to coast, and TELUS and YBVR have shown that they understand the popularity of soccer among Canadian families and sports fans, at a time when Canadian soccer is reaching new heights – not just at home but at a continental and global level. We’re delighted to join the likes of Real Madrid and Wimbledon in engaging viewers through the latest in technological innovation.”
The TELUS Sports app is available as a pilot for fans to watch the remaining nine matches in the season, for a total of four regular season matches and the five post-season fixtures which will determine the 2022 CPL champion.
About YBVR
YBVR is a technology company that is re-defining the way fans experience live sports. YBVR enables a new immersive and interactive dialogue with sport fans, especially the new generations, engaging from home and in-venue. The expansive expertise of these companies brings about a fantastic opportunity for the Canadian Premier League and its fans.
About MEDIAPRO Canada
MEDIAPRO Canada, the Canadian subsidiary of GRUP Mediapro, the Spanish media multinational, holds a long-term partnership with Canadian Soccer Business for the worldwide media rights to the Canadian Premier League, the country’s first-division men's professional soccer league; the Canadian Championship, Canada Soccer’s domestic soccer competition involving all professional soccer clubs in Canada including Major League Soccer’s Toronto FC, CF Montréal and the Vancouver Whitecaps; and the home friendly matches of Canada Soccer’s Women’s and Men’s National Teams. In 2019, it launched OneSoccer, a dedicated soccer channel available through TELUS Optik TV, fuboTV and the OneSoccer app.
About TELUS
TELUS (TSX: T, NYSE: TU) is a dynamic, world-leading communications technology company with $17 billion in annual revenue and 17 million customer connections spanning wireless, data, IP, voice, television, entertainment, video, and security. Our social purpose is to leverage our global-leading technology and compassion to drive social change and enable remarkable human outcomes. Our longstanding commitment to putting our customers first fuels every aspect of our business, making us a distinct leader in customer service excellence and loyalty. The numerous, sustained accolades TELUS has earned over the years from independent, industry-leading network insight firms showcase the strength and speed of TELUS’ global-leading networks, reinforcing our commitment to provide Canadians with access to superior technology that connects us to the people, resources and information that make our lives better.
TELUS Health is Canada’s leader in digital health technology, improving access to health and wellness services and revolutionizing the flow of health information across the continuum of care. TELUS Agriculture provides innovative digital solutions throughout the agriculture value chain, supporting better food outcomes from improved agri-business data insights and processes. TELUS International (TSX and NYSE: TIXT) is a leading digital customer experience innovator that designs, builds, and delivers next-generation solutions, including AI and content management, for global and disruptive brands across high-growth industry verticals, including tech and games, communications and media, ecommerce and FinTech, healthcare, and travel and hospitality. TELUS and TELUS International operate in 25+ countries around the world.
Driven by our determination and vision to connect all citizens for good, our deeply meaningful and enduring philosophy to give where we live has inspired TELUS, our team members and retirees to contribute more than $900 million and 1.8 million days of service since 2000. This unprecedented generosity and unparalleled volunteerism have made TELUS the most giving company in the world. Together, let’s make the future friendly.
For more information about TELUS, please visit telus.com, follow us @TELUSNews on Twitter and @Darren_Entwistle on Instagram.
CAN Health Network Provides National Access to Verto's Canadian-Made Digital Twin Technology
Verto's innovation accessible to national network of almost 30 healthcare organizations
TORONTO, Oct. 6, 2022 /CNW/ - After a successful commercialization project funded by the Coordinated Accessible Network (CAN) Health Network, Unity Health Toronto is implementing technology from Toronto-based digital health company, Verto, to help manage ambulatory patient flow, streamline consent management, and transform patient experience across dozens of ambulatory clinics.
Verto's Digital Twin Orchestration technology connects relevant pieces of information about patients across any IT system to build complex, dynamic profiles of each individual. The profile is then used to craft personalized care journeys, automating and orchestrating better experiences for individuals throughout their care.
"We are proud to deliver a solution that transforms Unity Health's patient experience," said Michael Millar, CEO & Founder of Verto. "Being 'powered by Verto' means we put control back in our clients' hands to innovate around challenges of legacy systems and workflows. In collaboration with CAN Health Network and Unity Health, we continue to build on our success with global consent management, vaccinations, and outpatient clinic engagement and are excited to accelerate our efforts to reach all of Canada."
Unity Health began working with Verto in 2018 to co-design solutions to improve patient experience and clinic flow across the organization. Last year, the organization realized the need to find novel ways to better coordinate and engage with patients before, during, after and in-between hospital visits.
"We were seeking a tool to support care pathway management and enhance the patient experience," said Frank Garcea, Executive Director, Information Technology, Unity Health Toronto. "A digital twin solution, like the one developed by Verto, allows us to do this, making sure that patients receive key information and engagement at appropriate times in their care pathway, while providing them with tools to better manage their clinic visits and interactions."
"The technology also supports the dissemination of digital surveys, which allow us to capture the patient experience in near real-time and then support immediate improvements to their experience," he said.
In 2020, Unity Health joined the CAN Health Network and launched a commercialization project with Verto to develop and implement a global consent model with a focus on digital communications. CAN Health Network's goal is to support the Canadian economy by identifying and introducing Canadian-made solutions in the healthcare marketplace.
Through commercialization projects like the one launched by Unity Health, companies like Verto get access to real-world environments, work closely with clinicians to validate their solutions, and benefit from the Network's unique procurement process.
On project completion, Unity Health launched a formal and competitive procurement process, ultimately selecting Verto as the best solution for their needs. As part of Unity Health's contract with Verto, all listed CAN Health Network members were given access to the solution without having to launch their own procurement process. This includes 27 healthcare organizations across Canada, comprising of health authorities and long-term care homes.
"This is what the Network is all about – validating Canadian technology solutions and making it easier for healthcare organizations to procure them," said Dante Morra, Chair, CAN Health Network. "Through leading-edge companies like Verto, we have the ability to solve our own problems with solutions made right here at home, benefitting all Canadians."
"Verto is another example of a fantastic Canadian company that has taken advantage of the CAN Health Network to deliver a solution that will help Canadians and improve patient experience. It's why we invested $30 million in the CAN Health Network in Budget 2022, to provide Canadian companies across the country even more access to a large, domestic marketplace where companies can scale up and be anchored in Canada," said Honourable Mary Ng, Minister of International Trade, Export Promotion, Small Business and Economic Development.
About Verto Inc.
Verto Inc. is a rapidly growing Canadian digital health company offering a Digital Twin Orchestration Platform. Our patented machine learning technology consolidates data from nearly any technology or database to create normalized, contextual snapshots – 'Digital Twins' – of each patient's healthcare journey. Verto then automates actions across different systems while securely managing consent, data, and communication. Our team consists of health informaticians, technologists, and clinicians who are passionate about creating bold and disruptive solutions to help every person reach their best personal health outcome. Learn more at https://verto.health
About Unity Health Toronto
Unity Health Toronto, comprised of St. Joseph's Health Centre, St. Michael's Hospital and Providence Healthcare, works to advance the health of everyone in our urban communities and beyond. Our health network serves patients, residents and clients across the full spectrum of care, spanning primary care, secondary community care, tertiary and quaternary care services to post-acute through rehabilitation, palliative care and long-term care, while investing in world-class research and education. Learn more at www.unityhealth.to.
About CAN Health Network
The CAN Health Network is a Canada-first approach to technology adoption. It helps break down barriers to scaling in the health-care system and provides an environment for companies to scale to their full potential. Currently operating in Ontario, Western and Atlantic Canada, the CAN Health Network was recently awarded $30 million by the Government of Canada as part of Budget 2022 to expand into Quebec, the Territories and with Indigenous Communities. Learn more about the CAN Health Network at www.canhealthnetwork.ca.
