| PEAR Sports Is Selected by Myodetox to Create a Custom Physical Therapy Mobile Platform to Further Elevate the Customer Experience Allows Therapists to Send Personalized Workout & Movement Exercises with Video and Audio to Support Client Health and Wellness NEWPORT BEACH, CA - December 4, 2019 - PEAR Sports, creators of the PEAR Health & Fitness Platform that delivers smarter digital coaching technology, today announced it has partnered with Myodetox to develop a custom digital exercise platform to build and deliver personalized coaching and therapy sessions to improve client treatment results. Myodetox is a group of lifestyle physical therapy clinics delivering a longevity-focused and personalized treatment approach for pain relief and body optimization. Myodetox is evolving the physical therapy (PT) market in response to growing consumer demand for preventive healthcare and intelligent recovery options. The company is delivering the first consumer brand experience in the PT industry by following best practices found in boutique fitness and luxury retail. The new Myodetox Therapist App by PEAR will extend the reach of expert therapists by providing personalized treatment exercises with rich video and audio coaching to clients on their mobile devices and wearables.Myodetox Founder & Chief Creative Officer Vinh Pham said, “We are very excited to work with PEAR Sports to deliver our unique therapy and movement programming to our clients in the convenience of a mobile platform. With the Myodetox Therapist App by PEAR, our expert therapists can customize a digital treatment plan for each client so he or she can reach his or her goals as quickly as possible. This new digital solution effectively extends the clinic experience and empowers our clients with more education and therapy programming to continue progress between sessions.” Bob Allison, Founder, PEAR Sports, said, “Myodetox is appealing to the lifestyle-driven consumer who seeks out an elevated therapy experience for their best health and well-being. It is gratifying for PEAR Sports to work with a brand that shares our focus on delivering authentic fitness and therapy content. The Myodetox Therapist App by PEAR will optimize client results by delivering therapy exercises in a rich mobile app that clients can use anytime, anywhere.”PEAR is expert at delivering personalized, adaptive coaching programs with bespoke content using mobile and wearable technology with human coach guidance and symbiotic music. Our digital coaching solutions include Workout Builder, a tool that allows enterprises like Myodetox to build and deliver customized fitness and therapy workouts for individuals and groups. With communication tools that support personal audio and video feedback, the Myodetox Therapist App by PEAR encourages compliance and the best results for both the clinic and the customer.To learn more about how PEAR’s digital coaching and wellness solutions can increase user engagement for your business, please visit www.pearsports.com. About PEAR SportsPEAR Sports is perfecting the personalized delivery of digital health, wellness and fitness programs. Our platform and solutions deliver on-demand customized coaching that creates great experiences for customers and enterprises. For more information, visit www.pearsports.com.About MyodetoxMyodetox is a group of lifestyle Physical Therapy clinics delivering a longevity-focused and personalized treatment approach for pain relief and body optimization. The Company is evolving the physical therapy market by delivering the first consumer brand experience in the physical therapy industry by following best practices found in boutique fitness and luxury retail.ContactsPEAR SportsAnita Habeich, anita@pearsports.comMyodetoxAaron Scheinfeld, aaron@myodetox.comShare This PEAR Sportshttps://twitter.com/PEARsportshttps://www.facebook.com/PearSports/https://www.instagram.com/pearsportshttps://www.linkedin.com/company/pear-sports-llchttps://pearsports.com/ Myodetoxinstagram.com/myodetoxfacebook.com/myodetoxmyodetox.com |
Author: trainitright
HOW TO AVOID ILLNESS AND OTHER MEDICAL CONSEQUENCES WHILE TRAVELING THIS CHRISTMAS

A record-breaking 112.5 million people – more than a third of all Americans - are expected to travel this holiday season, including 102 million who will take their trips by car. Nothing ruins a long-awaited vacation faster than getting sick or being in medical distress. We turned to Dr. Niket Sonpal, an NYC internist and gastroenterologist for some tips on how to avoid health consequences will traveling.
Avoid Deep Vein Thrombosis
Deep vein Thrombosis (DVT) occurs when a blood clot (thrombus) forms in one or more of the deep veins in your body, usually in your legs. Deep vein thrombosis can cause leg pain or swelling, but also can occur with no symptoms. For travelers, this can happen on long haul trips where you are not moving. Dr. Sonpal recommends if you are flying or on a train, to move around the cabin to get the blood flowing in your legs. If you are driving, take a break at a rest stop and walk around. Compression socks are also another option to prevent DVT.
Don’t Get Nauseous
People can experience motion sickness on virtually any mode of transportation. To combat this, Dr. Sonpal suggests Dramamine® Non-Drowsy Naturals, Dramamine®'s first non-drowsy formulation. It contains the clinically tested ginger dosage required for preventing and treating motion sickness. Other sources of ginger, including candies, gums, or ginger ale, may not contain a full clinical dose. For someone who is already experiencing nausea while traveling, it is a good idea to keep Emetrol on hand which is an over the counter nausea medication that does not cause drowsiness.
Avoid Bloating on a Plane
If you get gassy on a plane, you’re not alone! Dr. Sonpal explains that, “As the pressure around you decreases, the gas in your belly isn’t constrained as much and it expands. This can make you feel bloated or become distended.” It is essential to avoid foods that cause gas or have salt. Skip the tomato juice in flight and stick with non-carbonated water. Avoid alcohol, cruciferous vegetables, dairy and high sodium snacks such as salted peanuts or pretzels. Foods that are protein packed, magnesium-rich and high in Vitamin C are good options.
Sanitize Your Surfaces
Planes and trains are a breeding ground for illness. The former are awful due to re-circulated air. Most travelers would be appalled if they really knew how germy their tray tables are! Dr. Sonpal suggests sanitizing wipes for your tray table, seat belt clip and hand rests of your seats on planes and trains. When you exit a restroom on a plane or train and touch the door handles, be sure to use hand sanitizer even if you already washed your hands.
Get Your Shots Before Traveling Abroad!
Before you even book your trip, make sure you’re up to date on your shots. If you’re traveling to an area where you’re at risk for picking up an illness like malaria, you might be prescribed preventative medication. Dr. Sonpal suggests that, “People should use the CDC website for recommended vaccines for travel abroad or see a travel clinic. The health risks posed to Americans vary based on the country they are traveling to.”
Don’t Touch the Ice!
When traveling to a different country, most people are very cautious about only drinking bottled water. Many folks forget that ice is simply frozen water and put it into their soft drinks or alcoholic beverages. Contrary to what one might think, freezing water does not kill bacteria. The only way to be sure it is safe is if you boiled the water and then froze it.
Avoid Jet Lag
Even a relatively short time change from EST to PST (3 hours) can cause jet lag. With some international travel from the United States, the time difference can be as much as twelve hours. If you're traveling east, try going to bed one hour earlier each night for a few days before your departure. Go to bed one hour later for several nights if you're flying west. If possible, eat meals closer to the time you'll be eating them at your destination. Dr. Sonpal suggests that you set your watch to the new time before you leave. Once you reach your destination, try not to sleep until the local nighttime, no matter how tired you are.
Essential Medicine/Supplies to Travel With
Dr. Sonpal stresses never to check your medication with your baggage, always keep it in your carry on. Have a fresh re-fill on prescription medication with extra doses in case you get stuck at your destination. In addition, here are some essential over the counter meds/supplies to travel with:
Benadryl- For allergic reactions such as insect or bee bites.
Pepto Bismol- For diarrhea
Laxative such as Dulcolax
Anti-biotic ointment such as Neosporin
Common cold/sinus remedies such as Mucinex or Claritin
Pain relievers such as Tylenol or Motrin
Anti- Nausea medication such as Emetrol
Motion Sickness medication such as Dramamine. If you are the driver, be sure to take the non-drowsy version.
Electrolyte tablets for dehydration
Hydrocortisone cream to relieve itching from rashes, bites, poison ivy etc.
Aloe to soothe sunburned skin
Band-Aids
Digital Thermometer
Nasal Spray to prevent clogged ears while flying
Tweezers
Eye Drops
Epi-Pen if you are prone to severe allergic reactions
How to Find a Reliable Physician if you are Traveling Abroad
The US embassy in your destination country (http://www.usembassy.gov/) can help you locate medical services and will notify your family and friends in the event of an emergency. When selecting a doctor, make sure that he or she can speak your language. The following resources provide lists of doctors and clinics that can care of travelers:
The International Association for Medical Assistance to Travelers (www.iamat.org; membership required, but it is free)
Joint Commission International (www.jointcommissioninternational.org)
The International Society of Travel Medicine (www.istm.org)
Travel Health Online (www.tripprep.com; gets information from various sources so quality is not guaranteed)
Dr. Niket Sonpal is a native of Long Island NY and a graduate of Medical University of
.
.
He is the co‐author for the best-selling Master the Boards: USMLE Step 2 CK, Master the Boards Step 3, And Master the Boards: Internal Medicine.
Dr. Niket Sonpal is a native of Long Island NY and a graduate of Medical University of Silesia – Hope Medical Institute in Poland. After completing his residency in Internal Medicine at Lenox Hill Hospital, he was selected to be the 2013‐2014 Chief Resident at Lenox Hill Hospital–Northshore LIJ Health System. He is an Adjunct Assistant Professor at Touro College of Osteopathic Medicine and Clinical instructor at Kingsbrook Jewish Medical Center, Brooklyn.
Dr. Sonpal has completed his Fellowship in gastroenterology & hepatology at Lenox Hill Hospital and will continue his work in the field of medical student and resident test preparation. He now serves as the associate program director for the Internal Medicine Residency Program at Brookldale University medical center.
He is the co‐author for the best-selling Master the Boards: USMLE Step 2 CK, Master the Boards Step 3, And Master the Boards: Internal Medicine.
Flu Activity Currently Low in the US but Cases Likely to Rise, says GlobalData
Seasonal flu activity currently remains low in the US. However, leading data and analytics company GlobalData estimates that many more cases of influenza will occur this season, with more than 16.6 million diagnosed incident cases of influenza-like illness (ILI) and nearly 1.4 million laboratory-confirmed cases predicted.
Topias Lemetyinen, Managing Epidemiologist at GlobalData, comments: “Seasonal influenza is a contagious respiratory viral illness with symptoms such as fever, cough, sore throat and muscle aches and pains. Despite being a mild disease in most instances, in certain risk groups, influenza can cause serious illness. GlobalData estimates that near 8% of diagnosed incident cases of ILI in the US will test positive and be confirmed as influenza in 2019.”
“The analysis by GlobalData captures the flu season spanning from fall 2019 to spring 2020 in its forecast for influenza cases in 2019. Despite the current lull in flu activity, it is likely that many more cases of the flu will begin to appear before the end of this year, and the number will continue to grow into next year.
“Even though flu activity has remained low, we should not be lulled into a false sense of security as cases may begin to emerge. In order to best protect the groups most at risk, all those able to receive a vaccine should.”
PepsiCo and Hostess Brands’ snacks M&A shows consumers want health and indulgence, says GlobalData
Consumer interest in the links between diet and health is a key driver of the global snacks market, but so is their desire to treats themselves – as has been underlined by two acquisitions in North America, argues GlobalData.
Twinkies owner Hostess Brands has recently announced the purchase of Canadian biscuit business Voortman Cookies. This deal was then followed by PepsiCo unveiling its move for fellow US snacks supplier BFY Brands.
Dean Best, Food Editor at GlobalData, says: “Growing consumer awareness in the links between diet and health is reshaping the snacks industry in markets around the world. PepsiCo, home to Lay’s crisps and Doritos tortilla chips, buying better-for-you snacks firm BFY Brands, the owner of the low-calorie PopCorners snack, underscores that trend.
“However, consumers don’t want to eat healthier snacks all of the time; they want to treat themselves. Voortman has a range of healthier wafer products, opening up Hostess to a line of better-for-you products, but the deal further bolsters the US group in the indulgent end of the market, too, adding snacks such as chocolate-chip and fudge-striped almonette cookies.
“The snacks market is a clear example of bifurcation – with health and indulgence in growth. Expect more deals at both ends of the spectrum.”
Research in sheep suggests possible early test for fetal heart health
UNDER STRICT EMBARGO UNTIL 01.00 GMT THURS 5 DECEMBER 2019
Not for publication or broadcast before this time
Changes in heart rate, due to low oxygen conditions, experienced by the fetus during pregnancy, could be used to predict the future heart health of babies, shows research published in The Journal of Physiology today.
Previous research has shown that sustained low levels of oxygen (called chronic hypoxia) during pregnancy significantly impair the growth of the fetus, leading to a condition known as intrauterine growth restriction (IUGR). Chronic hypoxia during pregnancy can also impair the development of key organs, imposing consequences for life-time health risks, such as an increased heart disease risk.
The main causes of low oxygen during fetal development are complications with the placenta. Researchers at the University of Cambridge and the University of Washington led by Dino Giussani and Martin Frasch modelled such conditions during pregnancy by placing pregnant sheep in an environment with lower than normal oxygenation, similar to experiencing high altitude. They then looked at the fetal heart separated from any influences of the rest of the body, as a way to see if the heart shows any sign of “remembering” the effects of lower than normal oxygen that was experienced in the womb.
The way that they determined this was by looking at something called heart rate variability, which refers to changes in heart rate that normally occur in our hearts. Analysis of the patterns of these changes in heart rate were able to tell them if the hearts of the fetuses were impacted in the long term.
This research may have important clinical implications. If babies from complicated pregnancies are identified to have abnormal fetal heart rate patterns, then doctors could follow the development of these children more closely to better protect against any future risk of heart disease.
Martin Frasch, first author on the study said:
“We are excited about these results and the ability it may give clinicians to assess in a new way the hearts of kids who don’t show symptoms, who may have underlying heart problems. A good example of this would be kids in Brazil whose mothers were infected with the Zika virus. While they may not have symptoms, they may still actually have important problems with their hearts that we should be addressing.”
Notes for Editors
- Full paper title: First evidence that intrinsic fetal heart rate variability exists and is affected by hypoxic pregnancy https://physoc.onlinelibrary.wiley.com/doi/10.1113/JP278773 (link will only work after the embargo date. Before then, please email the press office for a copy of the paper)
- The Journal of Physiology publishes advances in physiology which increase our understanding of how our bodies function in health and disease. http://jp.physoc.org
- The Physiological Society brings together over 4,000 scientists from over 60 countries. The Society promotes physiology with the public and parliament alike. It supports physiologists by organising world-class conferences and offering grants for research and also publishes the latest developments in the field in its three leading scientific journals, The Journal of Physiology, Experimental Physiology and Physiological Reports. www.physoc.org
HOW TO GET RID OF HOLIDAY HANGOVER FACE
Tis' the season to be jolly. You partied and drank too much the night before and perhaps the one before that. Today, you have a business meeting or brunch with the inlaws. Although you might feel like you want to crawl into a hole from your hangover, you can’t afford to look like you have one. What is your skincare SOS game plane? We turned to Dr. Manish Shah, a Board-Certified Denver, Colorado Plastic Surgeon for some tricks that will have you looking fresh as a daisy even if they don’t cure that queasy feeling and headache.
Remove your makeup the night before
This is a tough one. If you come home stumbling at 3am, this is probably the last thing on your mind. If you can manage to do it, you’re one step ahead of the game. If going to the sink is too much effort, use cleansing cloths such as e.l.f. Cosmetics Hydrating Water Cleansing Cloths. They easily remove makeup and help nourish the skin with purified water. Infused with aloe and Vitamins B & E, the materials cool on contact and soothe and moisturize skin.
Hydrate
The number one thing alcohol does is dehydrate your entire body, including your skin," says Dr. Shah. "When skin is dehydrated it looks sallow, fine lines are more noticeable, and pores become obvious."
Dehydration is the main reason your skin looks terrible when you wake up. While drinking water is wise, the effects of that internal hydration won't immediately show up externally, says Dr. Shah. For a quick fix, he suggests a sheet mask that contains hyaluronic acid, a molecule that draws moisture to the skin. Try: Estee Lauder Advanced Night Repair Concentrated Recovery PowerFoil Mask
Put your face on ice
Skin redness can be caused by alcohol dilating your blood vessels. One way to reduce this is to put a cold compress on the affected area for a few minutes. But be warned, extended periods of drinking can cause permanent redness and skin damage.
Jade Rollers or Massage
You can use any jade roller (put it in the freezer for a few minutes for a cooling effect) or even just your fingers. Dr. Shah says that the goal is to energize and lift the skin by promoting lymphatic drainage (aka de-puffing).
De-Puff Your Eyes
After a night or two of drinking, the eyes become bloodshot because tiny blood vessels on the eye surface become red and inflamed.” Also, the diuretic effects of alcohol can lead to dehydration, which tells your body to hold onto more water weight. That fluid retention also prompts next-day puffiness.
You can purchase a product such as Skyn Iceland hydro cool firming eye gels. If you don’t have something like this in the house, you can substitute chamomile tea. Steep the tea bags in hot water, take them out until they cool, and put one on each eye for 10 minutes.
Exfoliate
Dr. Shah recommends giving your skin a good but gentle scrub. "Exfoliate off sallow, alcohol-damaged skin cells. You do not need to break the bank to do this. Dr. Shah suggests: St. Ives Invigorating Face Scrub – Apricot. This product can be found online and at most drug stores.
Seal Your Skin With A Spritz- And Not Champagne
After you have done all of the “damage control” you can give you face a final spritz of moisture with a product like Fresh Vitamin Nectar Antioxidant Glow Water. This energizing spray is packed with skin-brightening nutrients derived from citrus fruit, plus a blend of magnesium and zinc, to help snap sleep-deprived and hungover skin into shape.
Dr. Shah cautions that he does not endorse habitual binge drinking or alcohol abuse. These tips are for those who have overindulged on occasion. Aside from the obvious mental and physical effects of alcohol abuse, it can also cause harmful long term consequences to the skin which cannot be “fixed” by the tips given above.
Dr. Shah is not paid or in any manner affiliated with the products he mentions.
About Dr. Manish Shah
About Dr. Manish Shah
Manish Shah, M.D., F.A.C.S. was born in Canada and raised in the Washington, D.C. area. He graduated with honors from the University of Pennsylvania, receiving a degree in biomedical engineering. He then completed his medical training at the University of Virginia, earning his Medical Doctorate. During this time, he also completed a one-year fellowship in microsurgery research at the New York University School of Medicine / Institute of Reconstructive Plastic Surgery. As a prelude to his plastic surgery training, Dr. Shah completed a rigorous five-year training program in General and Trauma Surgery at Emory University and the Medical College of Georgia. His formal training in Plastic and Reconstructive Surgery was completed at the Univ. of Tennessee College of Medicine – Chattanooga Unit. After completing his plastic surgery training, he moved to New York City when he was selected for the prestigious Aesthetic Surgery Fellowship at Manhattan Eye, Ear, and Throat Hospital. He underwent extensive, advanced training in aesthetic surgery of the face, breasts, and body at the hands of some of the most renowned cosmetic surgeons in the world. This fellowship is widely considered to be the best of its kind in the world. Dr. Shah is one of only a select few plastic surgeons in the country who have undergone formal post-graduate training in aesthetic surgery.
Dr. Shah’s specialties include revision facial aesthetic surgery, rhinoplasty (“nose reshaping”), and aesthetic surgery of the breast (breast augmentation, breast lift, breast reduction). He is, however, well-trained in all areas of aesthetic surgery.
Dr. Shah’s aim is to obtain a natural appearing transformation that complements the real you!
Dr. Shah is a Clinical Assistant Professor of Surgery at the University of Colorado Health Sciences Center. He maintains a private practice in Aesthetic Plastic Surgery in the Cherry Creek neighborhood of Denver.
Dr. Shah is a member of the American Society of Plastic Surgeons, the American Society of Aesthetic Plastic Surgery, the International Society of Aesthetic Plastic Surgery, the Rhinoplasty Society, and the European Academy of Facial Plastic Surgery.
Dr. Shah is board-certified by the American Board of Plastic Surgery.
New approach to treating cystic fibrosis could lower risk of lung transplants and death, RCSI study
DUBLIN, Dec. 3, 2019 -- A new approach to treating people with cystic fibrosis (CF) has been shown to reduce inflammation, which has the potential to reduce the need for lung transplants and lower the risk of death.
The study, led by researchers at RCSI (Royal College of Surgeons in Ireland), is published in the current edition of the American Journal of Respiratory and Critical Care Medicine.
CF is a genetic disease that affects about 1,300 children and adults in Ireland and 70,000 worldwide. The main cause of death in people with CF is lung disease, which is driven by severe inflammation and chronic infection in the airways.
While recent years have seen the emergence of several new therapies aimed at improving lung function and survival, the lack of effective anti-inflammatory and anti-infective treatments for these individuals continues to represent a significant challenge.
The researchers found that one of the most aggressive bacteria found in the lungs of those with CF caused certain immune cells to change their metabolism. This change caused the immune cells to produce a protein that causes more inflammation. They identified that high levels of the protein were associated with worse lung function and a higher risk of death or need for a lung transplant.
The team then used a small molecule called MCC950 to reduce levels of the protein in a laboratory model of CF. In addition to reducing inflammation, this also helped clear the lungs of bacteria. This marks the first time that researchers were able to stop this protein in CF in vivo by targeting cell metabolism, which could potentially lead to a new approach to treating inflammatory diseases like CF.
“This is an important first step to significantly improving patient outcomes for people with cystic fibrosis. While more testing is required before delivering this to patients, we believe these results are very promising and could make this molecule a candidate for clinical trials,” said Professor Gerry McElvaney, the study’s joint senior author and Professor of Medicine at RCSI.
RCSI researchers, including joint senior and corresponding author Dr Emer Reeves, carried out the study in collaboration with the University of Duisberg-Essen and the Trinity Biomedical Sciences Institute. The research was funded by the StAR (Strategic Academic Recruitment) MD Programme, which aims to transfer impactful research discoveries to clinical practice more quickly for the benefit of patients.
"Previously, people with CF had a very low life expectancy. Due to improvements in medical treatment, these individuals are now living longer. However, they still suffer from a very severe disease. We hope that this advancement can lead to further improvements in outcome, better quality of life and eventually a normal life expectancy for our patients,” said Dr Oliver McElvaney, the study’s lead author.
RCSI is ranked among the top 250 (top 2%) of universities worldwide in the Times Higher Education World University Rankings (2020) and its research is ranked first in Ireland for citations. It is an international not-for-profit health sciences institution, with its headquarters in Dublin, focused on education and research to drive improvements in human health worldwide. RCSI has been awarded Athena Swan Bronze accreditation for positive gender practice in higher education.
Avicanna to Supply UK Market with Cannabis-Based Medicinal Products Through Distribution Agreement with the LYPHE Group
/NOT FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR FOR DISSEMINATION IN THE UNITED STATES. ANY FAILURE TO COMPLY WITH THIS RESTRICTION MAY CONSTITUTE A VIOLATION OF UNITED STATES SECURITIES LAWS./
TORONTO, Dec. 3, 2019 /CNW/ - Avicanna Inc. ("Avicanna" or the "Company") (TSX: AVCN) (OTCQX: AVCNF) (FSE: 0NN), a biopharmaceutical company focused on the development, manufacturing and commercialization of organic and sustainable plant-derived cannabinoid-based products, is pleased to announce that the Company has entered into an importation and distribution agreement (the "Agreement") with Astral Health Ltd. ("Astral"), the operating subsidiary of the LYPHE Group Ltd ("LYPHE Group" or "LYPHE") to supply its cannabis-based medicinal products ("CBMPs") to patients in the United Kingdom under the MHRA 'specials' programme.

"We look forward to the opportunity to provide access to our cannabis-based medicinal formulations to a population and medical community that is seeing increased demand for quality evidence-based products, such as our proprietary research-backed formulations. Both the Astral and Avicanna teams are aligned on the long-term vision and more importantly are qualified to provide the medical community in the United Kingdom with safe, effective and superior cannabinoid-based solutions, education and training," stated Aras Azadian, Chief Executive Officer.
Dean Friday, Chief Executive Officer of LYPHE, commented "We are very proud to be partnering with Avicanna in bringing their products to the UK market. Avicanna are market-leaders in terms of product quality, and their research-backed formulations give the company a distinct advantage in the UK market".
Under the Agreement, Avicanna will supply Astral with CBMPs, and both Avicanna and Astral will work together to achieve key strategic milestones in the United Kingdom related to market activation, market access and safety. The parties will also work together to collaborate on medical education programs with a focus on safety, dose guidance and efficacy. The Agreement will allow Avicanna to begin generating revenue from a growing UK market, while providing patients with increased access to the life-changing medicines they require.
It is expected that the CBMPs will be prepared in Colombia using Avicanna's proprietary research-backed formulations, using cannabis extracts from Santa Marta Golden Hemp S.A.S., a subsidiary of Avicanna, and manufactured at the facilities of Avicanna's exclusive Colombian manufacturer, Altea Farmaceutica S.A. using Good Manufacturing Practices (GMP).
To the knowledge of the Company, it carries out its operations in compliance with all applicable laws in the jurisdictions in which it operates.
About Astral & LYPHE GROUP
Astral is a Europe focused importer and distributor of medical cannabis, and forms part of the LYPHE Group. LYPHE has developed a patient-access ecosystem, which has laid the foundations for the medical cannabis industry in the UK, and has enabled the company to become the market leader in the country's rapidly expanding market.
About Avicanna
Avicanna is an Ontario corporation focused on the development, manufacturing and commercialization of plant-derived cannabinoid-based products through its two main business segments, cultivation and research and development.
Avicanna's two majority-owned subsidiaries, Sativa Nativa S.A.S. and Santa Marta Golden Hemp S.A.S., both located in Santa Marta, Colombia are the base for Avicanna's cultivation activities. These two companies are licensed to cultivate and process cannabis for the production of cannabis extracts and purified cannabinoids including cannabidiol (CBD) and tetrahydrocannabinol (THC).
Avicanna's research and development business is primarily conducted out of Canada at its headquarters in the Johnson & Johnson Innovation Centre, JLABS @ Toronto. Avicanna's scientific team develops products, and Avicanna has also engaged the services of researchers at the Leslie Dan Faculty of Pharmacy at the University of Toronto for the purpose of optimizing and improving upon its products.
Avicanna's research and development and cultivation activities are focused on the development of its key products, including plant-derived cannabinoid pharmaceuticals, phyto-therapeutics, derma-cosmetics and Extracts (defined as plant-derived cannabinoid extracts and purified cannabinoids, including distillates and isolates), with a goal of eventually having these products manufactured and distributed through various markets.
Stay Connected
For more information about Avicanna, visit www.avicanna.com, call 1-647-243-5283, or contact Setu Purohit, President by email info@avicanna.com.
Cautionary Note Regarding Forward-Looking Information and Statements
Certain information in this press release contains forward-looking statements. Such statements include but are not limited to the source of extracts to be included in the CBMPs, the manufacturer of the CBMPs, the ability of Avicanna to import the CBMPs, the ability of Avicanna and Astral to achieve the strategic milestones contemplated in the Agreement and the ability of Avicanna to generate revenue from the Agreement. This information is based on current expectations that are subject to significant risks and uncertainties that are difficult to predict, including the risk factors set out under the heading "Risk Factors" in the Company's long form final prospectus dated July 8, 2019. Actual results might differ materially from results suggested in any forward-looking statements. The Company assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those reflected in the forward-looking statements, unless and until required by securities laws applicable to the Company.
SOURCE Avicanna Inc.
African Mission Healthcare Launches Campaign
to Aid Africa’s ‘Forgotten’ War Victims
NGO provides vital ‘life support’ for only major hospital for
1.3 million people in conflict-torn Sudan’s Nuba Mountains
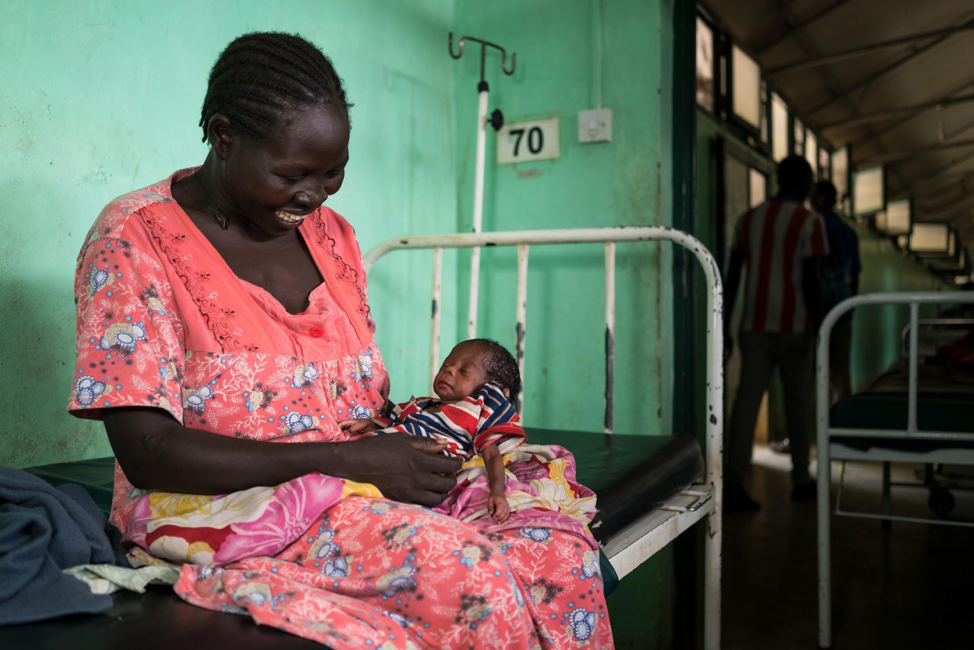
DELAND, Fla.—Aiming to provide vital medical help for hundreds of thousands of traumatized, but courageous and resilient victims of Sudan’s “hidden” war, U.S.-based nonprofit organization African Mission Healthcare (AMH, www.africanmissionhealthcare.org) launched a new campaign today—“Nuba 2020”— (www.Nuba2020.com) to coincide with Giving Tuesday, Dec. 3.
The Nuba 2020 campaign goal is to ensure the only major hospital in Sudan’s war-torn Nuba Mountains continues to save hundreds of thousands of children and adults threatened by conflict, disease and medical emergencies 365 days a year.
This is a once-in-a-generation opportunity to help the hurting and forgotten people of the Nuba Mountains before it’s too late,” said American missionary surgeon Dr. Tom Catena, who is leading the Nuba 2020 campaign and has been a medical missionary in Africa for nearly 20 years.
Catena—a native New Yorker and award-winning AMH-supported physician—is the only surgeon for 1.3 million people in the Nuba region, one of the poorest and most volatile places on earth. By contrast, the number of general surgeons needed to adequately serve the U.S. population is estimated to be at least seven per 100,000 people.
Shielded from much of the world, the little-known, yet catastrophic conflict in the Nuba Mountains—a region contested by the separate countries of Sudan and South Sudan—has claimed hundreds of thousands of lives and maimed and injured thousands more.
Situation Critical
Since 2008, Catena, a graduate of Duke University and former U.S. Navy doctor, has put his life on-the-line serving in war-zone Nuba, treating up to 350 patients a day—a staggering total of more than one million patients – including 2,200 direct war injuries, and saving the lives of tens of thousands of people.
During the war, most expat charity workers left, but “Dr. Tom,” as the locals call him, stayed. “I felt that if I left, I’d be saying my life was more important than theirs,” said 55-year-old Catena.
From 1983-2005, more than two million people died during the Sudanese civil war. After a six-year peace agreement, fighting erupted again in 2011, but now a tentative ceasefire is in place.
“The Nuba people are truly forgotten, caught in the center of the chaos and bombings, essentially stateless, and lacking any type of medical support from any government because they are stateless,” said Catena. “Without AMH’s support, thousands will suffer and die. We need to sustain the hospital and our network of clinics, buy new equipment and keep performing life-saving surgeries.”
In the shell-shocked Nuba region—roughly the size of Austria—Catena has kept the only major hospital in the region operating, even during bombing raids that targeted the hospital and his own home.
Against overwhelming odds, Catena and his team of local medical workers run the 435-bed Gidel Hospital and a network of six clinics, performing up to 2,000 operations, providing 53,632 hospital visits and caring for 80,000 patients at six community clinics annually on a total yearly budget of $1 million. By comparison in the U.S., the average number of general surgeries performed by male surgeons is approximately 500 cases a year.
“We’re the only facility in the Nuba Mountains where people can turn when their children’s lives are in jeopardy, they need surgery, or expectant mothers are having birth complications that too often can result in tragedy,” Catena said. Three out of every four women in the Nuba Mountains give birth without any medical assistance.
The Nuba 2020 campaign aims to raise $7.5 million to ensure the hospital and its network of clinics is able to continue its lifesaving work the next 20 years.
Nuba 2020: Keeping Hope Alive
“Without the hospital and clinics, hundreds of thousands of lives would be in peril,” said Catena. “But so many lives can be saved through essential medical care, and routine surgeries such as C-sections, that most Americans take for granted.”
While surgeries and hospital stays in the United States can cost tens of thousands of dollars, the same operations at Gidel Hospital cost a fraction of that. Further, according to Glassdoor, the average monthly salary for a U.S. nurse is approximately $6,000, compared to a nurse’s monthly paycheck in this region of just $265. The hospital relies entirely on charitable donations to cover costs.
“There are very few places on earth where so much can be achieved—and so many lives can be saved—with so little,” said Catena.
For more about the Nuba 2020 campaign, go to www.nuba2020.com.
About African Mission Healthcare
African Mission Healthcare (AMH, www.africanmissionhealthcare.org) is a Florida-based nonprofit organization, which strengthens African mission hospitals to aid those in greatest need. AMH strategically partners with mission hospitals to support and advance their commitment to provide compassionate, quality medical care to the forgotten and hurting people of Africa, and to improve the health system in sub-Saharan Africa. AMH was co-founded in 2010 by entrepreneur and philanthropist Mark Gerson and medical missionary Dr. Jon Fielder, who were college roommates.
# # #

