| SeabuckWonders Receives Best of Natural Beauty Award from Better Nutrition Sea Buckthorn Exfoliating Cleanser Wins in the “Natural Beauty Face” category—Here’s Why Chicago, IL – March 13, 2019 – SeabuckWonders’ Sea Buckthorn Exfoliating Cleanser has won the 2019 Better Nutrition Health + Beauty Awards in the “Face” category. Each year in spring, Better Nutrition magazine chooses the top health and beauty products from the natural products industry. Here’s why SBW’s facial cleanser stands out from the rest. SeabuckWonders exfoliating cleanser is specifically formulated for daily use, while other exfoliators can only be used a few times per week. SBW uses natural exfoliating beads made from jojoba esters that provide a very gentle grit. The gentle exfoliation is enough for a daily deep clean but not enough to cause the harsh abrasions that many cleansers do.Great for all skin types! Every skin type can benefit from exfoliation because it increases health by sloughing away dirt and dead skin cells. SBW uses a blend of sea buckthorn seed and berry oil, which provides nourishment to the skin. Sea buckthorn oil is incredibly balanced, and it helps bring balance to nearly every skin type.Not only does sea buckthorn oil feed the skin with rich omegas and antioxidants- it helps keep the skin supple and moisturized after exfoliation. SeabuckWonders Exfoliating Cleanser is Leaping Bunny Certified Cruelty-Free, Ashley KoffApproved and Vegan. Be sure to see all the winners on Betternutrition.com. All winners will be announced April 2019. |
| .About SeabuckWonders SeabuckWonders was the first company to introduce sea buckthorn to United States consumers. Providing only Himalayan, USDA Certified Organic Sea Buckthorn oil for over 20 years. Consumers can depend on the highest quality sea buckthorn oils when choosing SeabuckWonders. The SeabuckWonders Difference What separates their products from other brands? SeabuckWonders products contain the highest amount of sea buckthorn oil compared to competitors. SeabuckWonders products are available at select retailers or online at: http://www.seabuckwonders.com/products/ |
Author: trainitright
CAULIPOWER, THE BETTER-FOR-YOU PIZZA, NOW AVAILABLE IN 1000+ RETAILERS
LOS ANGELES, March 13, 2019 – CAULIPOWER®, delicious pizza that loves you back, has expanded to more than 1,000 retailers having launched only last year. Proudly made in Canada, CAULIPOWER is now available in freezer aisles across the country, including Loblaws, select Sobeys locations, Metro Inc., Longo’s, Walmart Canada, Whole Foods, Costco Wholesale, many independent retailers, and more to come throughout the year.
“We are delighted that CAULIPOWER is proving such a hit and it’s less than a year since we launched, “ said Gail Becker Founder and CEO CAULIPOWER. “People told us they wanted a frozen pizza that tastes like the real thing, and is better-for-you. With a cauliflower crust, our pizza really can be your new favorite vegetable.”
CAULIPOWER has reinvented pizza with a game-changing cauliflower crust that tastes like traditional thin crust pizza, but is more nutritious, with fewer calories and is naturally gluten-free. The brand disrupted both the U.S and Canadian market as the #1 better-for-you pizza option with Margherita, Three Cheese, Veggie, and Plain Cauliflower Pizza Crust varieties. CAULIPOWER pizzas are lower in calories, sugar, fat and sodium and higher in protein, fiber, and vitamins than most traditional and gluten-free pizzas.
Fans are encouraged to share their pizza moments on social with #PizzaLovesYouBack. For more information and to find a retailer near you, please visit:
About CAULIPOWER
CAULIPOWER ingeniously reinvents your favorite comfort foods to be lavishly nutritious, righteously delicious and accessible to all. CAULIPOWER products use real cauliflower and nothing synthetic, creating vitamin-rich and naturally gluten-free options that are lower in calories, sugar, fat, and sodium. Innovators and originators of a white-hot grocery category, CAULIPOWER is the #1 better-for-you pizza, #1 cauliflower crust pizza, #1 gluten-free pizza, and fastest growing pizza brand in North America. Founder, CEO and mom of two sons with Celiac Disease, Gail Becker left a global executive position to launch the company in 2017 after realizing that the food industry was in no hurry to offer effortless, healthy options that taste like the real thing. Every CAULIPOWER purchase benefits OneSun, a program installing edible teaching gardens in underserved public schools. CAULIPOWER is brought to you by Vegolutionary Foods, a family of convenient, veggie-forward, meal-hacking products. Find one of CAULIPOWER’s 20,000 retailers in North America and get recipe inspiration at eatCAULIPOWER.ca or join the @CAULIPOWERED community on social.
Venus Concept Launches Venus Heal™, an Innovative Treatment Modality for Soft Tissue Injuries
Introducing Venus Heal™, now cleared in Canada to treat acute and chronic musculoskeletal disorders, such as muscle spasms, back pain, and soft tissue injuries.
TORONTO, March 13, 2019 /CNW/ - Venus Concept Ltd., an innovative global medical technology leader, announces the launch of its latest medical device, Venus Heal™, in Canada. With over 11,000 systems installed worldwide, Venus Concept has expanded their suite of leading medical devices and incorporated their most advanced technology into this latest innovation.
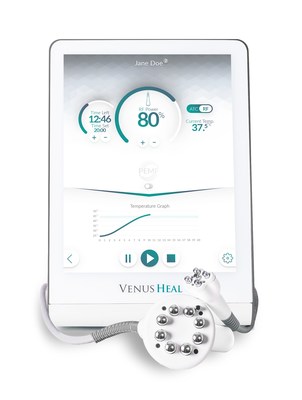
Venus Heal™ is powered by RP3 technology, which synergistically combines the benefits of Multi-Polar Radio Frequency (RF), Pulsed Electro Magnetic Fields (PEMF), and massage. RP3 works to increase tissue temperature, resulting in pain relief, myorelaxation, increase of local blood circulation, and reduced edema. In addition to its compact and portable design meant to adapt to a specialist's needs in-clinic or on-site, the device also includes advanced technological features for superior patient comfort and consistent treatment efficacy.
Treatments with Venus Heal™ have been helping Elias Theodoreau, world-ranked professional Mixed Martial Artist, to compete at the highest level in his sport. "Venus Heal™ played a very important role in my recovery from my last fight. It also allows me to accelerate recovery from the day-to-day grind that training puts on my body," says Theodoreau. "Luckily, with Venus Heal™ in my corner, I am able to compete at 100 per cent of my ability on fight night."
"We are thrilled to be announcing the launch of Venus Heal™ in Canada, namely because this state-of-the-art technology makes it an industry first for accelerated soft tissue healing," says Domenic Serafino, Chairman and Chief Executive Officer at Venus Concept. "Whether patients are athletes with acute injuries or workers with repetitive strain injury, Venus Heal™ offers the newest modality and the best solution to accelerate the healing of soft tissue injuries and conditions. Combined with Venus Concept's unique business model, our customers realize a higher ROI with low-risk ownership. It's the perfect solution for all rehabilitation professionals."
About Venus Concept® Ltd.
Venus Concept® is a leading global medical technology company that develops, commercializes, and delivers safe, efficacious, and easy-to-use non-invasive technologies and related practice enhancement services in a unique, industry-disruptive subscription-based business model. Venus Concept's devices have been designed in cost-effective and proprietary ways that enable the company to excel in multiple medical industries. The company has expanded its subscription platform and is now selling its devices in over 60 countries, including 27 with direct offices. Venus Concept has more than 400 global employees whose customer-centric approach has supported the company's rapid growth. For more information, please visit www.venusconcept.com.

SOURCE Venus Concept
New FDA Commissioner: What this means for Cancer Testing and Treatment
By Joshua Mansour, M.D.
The head of the National Cancer Institute, Dr. Norman “Ned” Sharpless, will be temporarily appointed as acting commissioner of the Food and Drug administration amid the news of Dr. Scott Gottlieb stepping down for personal reasons. Dr. Sharpless oversees billions in grants for research and federal funding at his current position. Time will tell if the appointment of this new commissioner or his successor will open up more dialogue regarding and addressing funding for research, expense of diagnostic and genetic testing, drug development, treatment regimens.
Cancer testing and cancer therapy continue to evolve at a rapid pace in hopes of helping as many patients as possible rid this terrible monster. Millions of people are affected by cancer and according to the International Agency for Research on Cancer, each year 18.1 million cancer new cases are diagnosed worldwide and approximately 9.6 million deaths occur from the disease. This number will only continue to rise with increased lifespan, improved diagnostics, and environmental influences. By 2030 these numbers are expected to grow to 21.7 million new cases and 13 million cancer deaths. This devastating illness continues to affect patients and their families in several ways. While these patients are struggling for their lives, at the same time many of them are struggling to pay the bills for their treatments and make ends meet. A typical cancer patient’s treatment can exceed $100,000 dollars a year with medical office bills, prescriptions, cancer treatments, and testing, in addition to the other miscellaneous items.
Billions of dollars globally are donated to cancer research yearly. However, only approximately 2% of this money will actually be used on developing a treatment that can be used in practice. In addition, much of the money that is spent on cancer research in pursuit of promising new treatments is unfortunately spent pursuing dead ends. In May 2016 the largest study of clinical development success rates showed that only about 5.1% of the treatments will even pass clinical trials. Although not all research will at first succeed, and this part of the process, what remains concerning is funding for projects that may make outlandish claims without scientific evidence to back them up.
This then raises another economic question - are eccentric assertions of the success of tests and treatments being made for financial gains for further additional funding? Recently a company participated in an interview where they have claimed to find the “cure for cancer” in one year. Although this may grab headlines, this statement is likely premature and does not have the backing yet for such a claim. Is it possible that the goal of such statements is to receive further funding for continued research and development and is this the best use of the money without more evidence? False positives and exaggerated results have reached alarming rates. There will have to be further due diligence and accountability prior to shelling out these research funds.
Lately, the development of a more personalized approach with genetic and mutation-specific testing has been implemented to determine if particular treatments will be beneficial to a patient. This precision testing has proved to be effective for certain cancers that may have germline and somatic alterations that can be targeted. In certain situations, approval of off-label drugs can be approved to treat the patient. While this can many times save a patient from unnecessary chemotherapy that may not benefit them or point them in a direction towards a regimen that will be more effective, it is unfortunately expensive to develop, run, and process and there is no guarantee that there is a treatment available that is specific to the patient’s disease.
Currently, the development of new cancer drugs that target specific pathways, molecules that interrupt signal transmission, activation/inhibition of proteins, antibody therapy, immunotherapy, and other cellular therapy have been rising. These therapies can stop cancer cells from multiplying, induce cellular destruction, or lead to a dynamic immune response to attack. Companies and organizations are investing more and more in these complex drugs that will hopefully decrease the need for older cytotoxic drugs that are designed to kill rapidly proliferating cells with little expense to the type of cell. This archaic (yet many times still effective) non-specific method may lead patients to experience severe adverse effects nausea, diarrhea, fatigue, and decreased cell counts requiring transfusions and hospitalizations.
The affordability of these new drugs that are being released raises another concern. Just because a new drug passes clinical trials doesn’t mean that it will be approved to be immediately administered, and cost plays a large part in this process. Analysis of the new cancer drug approvals by NICE shows that only about 51% of approved therapy is affordable for routine use and patients may not be benefitting from them. Ideally, physicians would be able to administer a drug that they believe would give their patient the best chance of survival with the least amount of suffering. However, this is not the case and the financial logistics concerning this can be improved just as much as the therapies we currently have to treat cancer.
At the end of the day, patients are living longer as their diagnosis is being made earlier and the treatments are improving. For good reason, money is being poured into the research and development of better tests and management. Virtually every person knows a person who has been affected by cancer, and collectively we are hopeful for continued evolution of treatment, care, and soon, a cure.
About Joshua Mansour, MD:
Dr. Joshua Mansour is a board-certified hematologist/oncologist working and in the field of hematopoietic stem cell transplantation and cellular immunotherapy in Stanford, California. Recently he has managed to have over 10 recent abstracts and over 10 recent manuscripts published in esteemed journals and given countless presentations at conferences and other institutions. He has helped design and implement clinical studies to evaluate current treatment plans, collaborated on grant proposals, and lead multi-institutional retrospective studies that have been published.
Precision BioLogic's CRYOcheck™ Factor VIII Inhibitor Kit Cleared for Sale in U.S.
HALIFAX, March 13, 2019 /CNW/ - Precision BioLogic Inc., a leading developer of hemostasis diagnostic products, is pleased to announce FDA 510(k) clearance and the launch of its CRYOcheck Factor VIII Inhibitor Kit in the U.S. This clearance follows approvals received from regulatory authorities in Canada, the European Union, Australia, and New Zealand, where the kit launched in February of this year.

The presence of factor VIII (FVIII) inhibitors reduces therapy effectiveness and is one of the most complex and costly complications for people with hemophilia A. Because of this, it is important for clinical laboratories to have a testing system that can accurately and precisely quantify FVIII inhibitors in patient samples.
Precision BioLogic developed the CRYOcheck Factor VIII Inhibitor Kit to address this challenge. It contains standardized components and a validated procedure to prepare patient samples for performing a modified Nijmegen-Bethesda assay as per the U.S.-based Centers for Disease Control and Prevention (CDC) recommendation1. A modified Nijmegen-Bethesda assay is used to determine FVIII inhibitor levels in people with hemophilia A.
According to Paul Empey, President & CEO of Precision BioLogic, FDA 510(k) clearance of the CRYOcheck Factor VIII Inhibitor Kit is an exciting milestone for the company but more importantly, a step forward for patient care. "Enhancing patient care is at the core of everything we do at Precision BioLogic," he explains. "Our kit will standardize the preparation of patient samples for inhibitor titer measurement. This, in turn, will help labs deliver accurate results for the diagnosis and routine monitoring of FVIII inhibitors."
To eliminate FVIII depleted plasma as a potential source of variant and standardize inhibitor titer measurement, the kit was developed with the following components:
- Imidazole Buffered Pooled Normal Plasma
- Imidazole Buffered Bovine Serum Albumin
- Negative FVIII Inhibitor Control
- Positive FVIII Inhibitor Control
Like all of Precision BioLogic's CRYOcheck products, kit components are frozen, allowing for fast and easy preparation.
About Hemophilia A and Inhibitors
Hemophilia A is an inherited bleeding disorder caused by insufficient clotting factor VIII (FVIII) in the blood. People with hemophilia A experience prolonged bleeding, which can lead to permanent joint damage and life-threatening hemorrhages. The standard treatment for people with hemophilia A without inhibitors is intravenous (IV) FVIII replacement therapy with recombinant FVIII (rFVIII) or plasma-derived FVIII (pdFVIII) concentrates. Prophylaxis, the regular infusion of clotting factor concentrates, is used to prevent bleeds thereby minimizing joint damage.
Unfortunately, up to 30% of people with hemophilia A develop inhibitors, an immune response to treatment with clotting factor concentrates. Inhibitors make it more difficult to manage and treat hemophilia. In fact, according to the World Federation of Hemophilia, apart from access to care and treatment, inhibitors are the most serious challenge in hemophilia care today.2 While routine blood tests may suggest the presence of anti-FVIII antibodies, specialized testing is important to confirm not only the presence of inhibitors but also the quantitation to effectively adjust treatment. Current methods for inhibitor testing vary from lab to lab.
About Precision BioLogic
Precision BioLogic Inc. is a privately-held company that develops, manufactures and markets the CRYOcheck line of frozen products used by medical professionals and researchers around the globe to diagnose coagulation disorders. Precision BioLogic also has several active initiatives with pharmaceutical partners who seek to ensure that the diagnostic implications for their novel therapeutic agents have been well characterized. In November 2018, Precision BioLogic acquired Affinity Biologicals, enabling the company to expand its clinical and research offerings to include an extensive line of coagulation-related antibodies as well as other products and services. For more information, visit www.precisionbiologic.com.
1 Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM, the Hemophilia Inhibitor Research Study Investigators. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost 2012; 10: 1055–1061.
2 World Federation of Hemophilia. Current issues in inhibitors. Available at https://www.wfh.org/en/Current-issues-in-inhibitors. Accessed on January 21, 2019.
SOURCE Precision BioLogic
IMPLANTS AND BREASTFEEDING...
WHAT YOU SHOULD KNOW BY:
Stephanie Canale, M.D.
Founder, Lactation Lab
One topic of concern to some mothers with breast implants is whether they present any health risk to their baby through possible contamination of their breast milk. In particular, they ask about whether silicon, silicone gel or platinum used in implants can leak into their breast milk.
Silicon and Silicone
Moms should know that silicon occurs naturally. It is the second most abundant element in the earth’s crust. It is thought to be essential for connective tissues and is found in tendons, bone, skin, hair and nails. Silicon is used as an anti-foaming agent in fruit juice and is found in cosmetics, pharmaceuticals and proshtheses. The only known health hazard results from inhaling crystalline silica dust into the lungs causing silicosis. Silica dust is never used in implants.
Breast implants contain silicone gel, a synthetic polymer made up of silicon, oxygen and other elements, most typically carbon and hydrogen.
Research has reported cases of esophageal dysmotility (slow movement through the esophagus) in infants that were nursing from mothers with silicone implant). Another study reported “rheumatoid-like symptoms” transferred to a child from a breastfeeding mother with silicone implants). However, this study examined only two children.
A larger study did not show any adverse outcomes in infants of breastfeeding mothers with silicone implants. Another study found that there were actually higher levels of silicon in cow's milk infant formulas than in human milk. (It should be noted that this study was funded by the plastic surgery industry.)
The silicon-containing anti-colic agent simethicone has been widely used for decades and there have not been shown to have any toxic side effects to infants.The American Academy of Pediatrics therefore recommends that mothers with silicone breast implants should breastfeed if they choose to.
The American Academy of Pediatrics Committee on Drugs does not feel that the evidence currently justifies classifying silicone implants as a contraindication to breastfeeding.
Bottom line: There is very little reliable data about breastfeeding with implants. Given that silicon is so abundant in our environment, and that there are overwhelming advantages of breastfeeding we strongly encourage women with breast implants to breastfeed if they wish to.
PLATINUM
While the limited evidence available suggests there is little risk from silicon, this may not be the case with platinum, which is used in silicone and saline implants. Platinum is a toxic metal that is especially damaging to early development.
There have been some studies showing significant levels of platinum in silicone breast implant gel and envelopes. Platinum has also been shown to leak out of the implant and accumulate in the tissues of women exposed to silicone breast implants . The question then becomes should women with saline or silicone breast implants be concerned about platinum?
Our research has found only one study to date that looked at platinum in breast milk in mothers that had silicone breast implants. It included 18 women with silicone breast implants and 5 women without ( the control group). The authors examined blood, urine, hair, nails, sweat and the breast milk of these women.
The concentration of platinum in blood did not differ between mothers with exposure to silicone through implants and those without. Both groups did have detectable levels of platinum thought to be related to environmental exposure. Similarly the concentration of platinum in urine did not differ between mothers with exposure to silicone through implants and those without.
However, there was a significant difference in platinum concentration in the hair, nails, sweat and breast milk in the implant group versus the control group, where no platinum was detected.
The study concluded that women with silicone breast implants had much higher levels of platinum than women without implants whereas saline implants did not contain any platinum. However, the methodology of the study has been questioned.
Bottom Line: Due to the small sample size, more research needs to be done to replicate these findings. However, it does raise some questions about platinum in breast milk. If mothers are concerned about platinum or silicone in their breast milk, Lactation Lab offer custom test kits to measure the levels of the components of their milk, along with recommendations for how to address any concerns.
KushCo Holdings, Inc. to Offer Certified Biodegradable, Compostable and Sustainable Packaging Solutions for the Cannabis Industry
GARDEN GROVE, Calif., March 12, 2019 – KushCo Holdings, Inc. (OTCQB: KSHB) (“KushCo” or the “Company”), in line with its previously announced sustainability initiatives, today announced it has signed a long-term development and distribution agreement with IEKO Corporation (“IEKO”), for the production of compostable and biodegradable packaging products for use in the cannabis and CBD industries.
Pursuant to the agreement, IEKO will work with KushCo to develop formulations and products designed for the unique demands of the cannabis and CBD industries, while ensuring that all new products are environmentally friendly by featuring proprietary, biodegradable materials from renewable resources. All packaging solutions produced under the agreement will be tested using ASTM and BPI standards to guarantee proper certification. Additionally, IEKO will provide dedicated research and development, quality control and account management personnel.
KushCo Holdings’ Chief Executive Officer, Nick Kovacevich commented, “It’s our responsibility to reimagine our products today to ensure the viability of our planet tomorrow. Customers are demanding an environmentally conscious solution for their everyday packaging needs. Partnering with Bob Meers and IEKO is a huge win for us. It’s inspiring to see the former CEO of Reebok and Lululemon so impassioned about the environment and I’m honored to be a part of the eco-sustainability movement our customers are yearning for. With a demonstrated ability to design and produce ground-breaking products, we are confident in the capabilities of the IEKO team and look forward partnering with one of California’s top packaging engineers.”
Robert J. Meers, Chief Executive Officer, “Our partnership allows us to be a vertically-integrated formulating, compounding, processing, and producing solution committed to making 100 percent commercially compostable, sustainable packaging without exception. Partnering with KushCo gives us the ability to continue to develop the next generation of renewably resourced, zero-waste packaging materials and we are thrilled to be partnering with a company that shares our commitment to the IEKO mission of ‘do no harm.’”
To be added to the distribution list, please email ir@kushco.com with “Kush” in the subject line.
About KushCo Holdings, Inc.
KushCo Holdings, Inc. (OTCQB: KSHB) (www.kushco.com) is the premier producer of ancillary products and services to the cannabis and hemp industries. KushCo Holdings’ subsidiaries and brands provide, product quality, exceptional customer service, compliance knowledge and a local presence in serving its diverse customer base.
Founded in 2010, KushCo Holdings has now sold more than 1 billion units to growers, processors and producers across North America, South America, and Europe.
The Company has been featured in media nationwide, including CNBC, Los Angeles Times, TheStreet.com, Entrepreneur, and business magazine Inc. While KushCo Holdings provides products and solutions to customers in the cannabis and CBD industries, it has no direct involvement with the cannabis plant or any products that contain THC or CBD.
For more information, visit www.kushco.com or call (888)-920-5874
Vogelzang Law Raises Funds at American Lung Association’s Fight For Air Climb
Chicago, IL (March 12, 2019) —
Vogelzang Law made a strong showing over the weekend at the American Lung Association’s annual Fight for Air Climb. The climb, which takes place in the Presidential Towers in downtown Chicago, invites participants to climb 180 skyscraper floors to raise awareness and funding in the fight for better lung health.
Team Vogelzang Law successfully climbed all four towers this year, raising $934 between the law firm’s six participants. As a whole, the American Lung Association raised nearly $500,000 at the climb on March 10th. These funds will support educational initiatives, important research, and community outreach efforts on behalf of lung health. As a law firm completely dedicated to mesothelioma clients, Vogelzang Law knows the importance of clean air and healthy lungs. The team remains committed to a cure and hopes to continue to find ways to positively impact the Chicago community.
The 2019 Fight for Air Climb was a huge success in support of individuals and families facing lung disease. Vogelzang Law proudly supports the American Lung Association's mission to improve lung health and prevent lung disease.
###
About Vogelzang Law
Vogelzang Law is a boutique asbestos litigation law firm representing victims of mesothelioma. Backed by Lead Counsel Nicholas Vogelzang’s nearly 20 years of asbestos law experience, we have the knowledge and resources required to pursue complicated cases with compassion and wholehearted advocacy.
Feel Your Best: 4 Tips for Creating a Personalized Approach to Optimum Health

Health is a deeply personal subject that cannot be addressed with a one-size-fits-all solution. Each person is biologically different with varying needs based on their age, sex, race, socioeconomic status, lifestyle choices, and relationships. With that being said, it’s hard to believe that one expert has all the answers considering how wildly different each person is.
In order to feel your best, you’re going to want to take a personalized approach to optimum health. The following four tips will help you with your journey to wellness.
Develop a Dream Team of Healers
Your personal ‘Dream Team’ may consist of your physician, massage therapist, chiropractor, therapist, acupuncturist, and dietician. Having access to the best healers in the industry is advantageous. Life science data validationensures that the practitioner that you’ve chosen to work with is licensed. That way, you’re working with someone who is legally capable of helping you heal.
Learn to Listen to Your Body
The body gives us subtle clues all the time to how it’s feeling. For example, drinking a glass of water when a mild headache manifests can help combat dehydration, the source of your ailment. When things don’t feel right, see what you can do to make yourself feel better. If a pain or illness persists, contact your doctor immediately for an appointment.
Make Mental Health a Priority
With so much emphasis on physical health, it’s important to remember to tend to your mental health, too. Talk therapy is a big help. It allows you to air your grievances and work through difficult feelings as they come up in your life. Having a safe place to emote and express yourself benefits your overall health and wellness.
Identify Imbalances and Deal with Them Immediately
When something is off-kilter, you’ll know it. Learning to recognize the areas of your life that don’t feel right allows you to improve them and lower your levels of stress. You feel in control of your life and health because of your self-awareness and willingness to change what doesn’t sit well with you.Take into account the things that make you different and then find ways to make them work for you and your wellness routine. Your personalized approach to health is worth the effort. It’s something that resonates with you and a plan that you’ll stick to long-term. Your health will flourish the more you make y
WHAT ARE THE PROS AND CONS OF 2019’s TRENDY WORKOUTS?
A MILLENNIAL CERTIFIED TRAINER BREAKS IT DOWN
Just like fashion, music and interior design, fitness goes through fads. Who hasn’t seen an Insta post of “Goat Yoga?” For those who bopped around to Madonna, we can’t forget Jazzercise, step aerobics, The Thigh Master, Tae-Bo, The Abdominizer, Buns of Steel, and the list goes on. Just because all “the cool kids” are doing a certain workout, does not mean it’s right for you. We turned to Vince Sant, Certified fitness trainer with ISSA and CEO of Vshred.com a fitness platform. Vince certainly speaks from a place of knowledge, with close to 1 million YouTube subscribers tuning in for his advice.
Wearable Technology
Whether they’re fitness trackers, pedometers, or calorie counters, this market has reached scheduled 4 billion in sales as of 2017. One study showed that non-wearers experienced more weight-loss because they relied on personal effort and, literally, sweat, to guide their workouts vs. a numerical data indicator. Vince suggests that “Sweat and good old-fashioned fatigue may be more reliable than data numbers on your wrist. After a few weeks or months, the novelty of knowing your step totals or resting heart rate wears off--even faster if you know about inaccurate those numbers can be.”
Spinning
People choose spinning for a variety of reason including weight loss, cardio and stress relief. Spinning is great for weight loss if done correctly and with a sensible diet that produces a caloric deficit. Vince Sant cautions that ”Spinning excessively can actually lead to atrophy of the muscles in your legs which is the opposite of strengthening them. The simple truth is that losing weight without strength and weight training can and will lead to atrophied and weakened muscles throughout the body”. Although cycling helps build some muscle, doing excessive amounts of cycling without the addition of strength training is not a comprehensive well-rounded workout.
Boxing
Boxing is a great workout for your entire body. It has stress relief benefits, helps concentration and eye-hand coordination. The improved endurance and stronger muscles you build translate effectively into life outside the ring, giving you the ability to perform everyday movements with energy and a decreased risk of injury.
Vince points out that, “With contact, boxing injuries can be a factor. Even while wearing head and mouth protection, sparring and competition can lead to minor injuries such as bruises and split lips and more serious injuries such as broken bones and concussions.” Vince suggests limiting boxing trained to shadow boxing, striking focus mitts and the smaller focus pads and hitting the heavy bag and speed bag, along with classic boxing training modalities such as running and jumping rope.
HIIT Training
HIIT training is a type of metabolic workout where you use both cardiovascular and strength training. Your aerobic and anaerobic systems will both be used throughout the routine, meaning glycogen and fat will both be used as fuels. Most of these training sessions last between 10-40 minutes. Vince says that “The biggest risk of this type of training is an injury caused by lack of supervision or know-how of the person training.” Studies show that your resting metabolic rate can be increased for the 24 hours after you do these types of workouts and that it may burn fat more efficiently than long aerobic workouts. Vince says, “The key is to recognize signs of fatigue that can lead to poor form and injury.”
Metabolic Training
This is a combination of cardio and strength conditioning. As examples, think of P90X, CrossFit, Insanity, or high-intensity circuit training. Metabolic training is about increasing "the storage and delivery of energy for any activity." If you do this type of training the correct way, you can burn more calories, get ‘afterburn,’ build endurance, strength, and fitness while having variety. Since this type of exercise seems to have so many benefits, why doesn’t everybody just do it? “The problem,” says Vince Sant, “Is that it’s just too intense for many beginners.” The cons of this workout are that there is a high attrition rate, a risk of injury and muscle soreness. If you are set on doing this program, Vince advises to bone up on more basic fitness first.
Body Weight Training
These are simple, effective exercises to improve balance, flexibility, and strength without machinery or extra equipment. Examples include:
Push-ups
Pull-ups
Dips
Squats
Lunges
The pros of this method include high repetitions and increased endurance. There is no equipment required, it is convenient and can be done at home or while traveling. There is less risk of injury while working several muscle groups at once. Vince says, “Some of the cons include that it may be difficult to achieve an intensity that is near the individual’s one-rep max. It also may be difficult to execute for those who are overweight.”
Vibration Boards/Platforms
“These are an absolute no,” says Vince Sant. He adds, “Vibration boards do not, and will not, work better than traditional exercises like barbells or cardio. The theory is that the vibration signals are transferred into body tissues, tendons, and muscles, which increases muscle contractions and ultimately improves muscle strength, coordination, and balance. In the long term, such contractions would increase muscle mass and energy expenditure, leading to better control of blood sugar levels. But these are still theories. The overall effects of whole-body vibration therapy remain elusive, as scientific studies vary largely in the vibration parameters used.”
If you are confused, what is the best method to adopt for your workout?
Vince stresses that “a workout should be fun, convenient, not cause repetitive injury. It should involve a warm up and cool down, have appropriate gear, be mindful of weather, be aware of your body. If something is causing pain beyond burn, stop that exercise. Workouts should take into consideration pre-existing injuries. For example, if you have chronic knee pain, swimming is a good option, while jumping and squats are not. Vince sums it up with, “For the most well-rounded workout, be sure to incorporate, balance, aerobics, flexibility, and strength training.”
Vince Sant
Fitness Expert & Co-Founder V Shred
Vince Sant, 25, is the Co-Founder of V Shred, the fastest growing online fitness and nutrition portal in the world. As an ISSA Certified Fitness Trainer, Former
Model Turned Fitness Expert, Instagram Fitness Guru & YouTube Sensation,
Vince’s mission is to offer the masses a sustainable and transformative
lifestyle-based training program designed to put the fun back into fitness and
nutrition.
In 2015, Vince co-founded V Shred along with Nick Daniel, Roger Crandall, Kevin Pearn, who sought out to create a healthy fitness movement specifically designed to deliver profound changes in your body with the minimal amount of workout time.
Vince is responsible for creating some of the world's most popular fitness and nutrition programs such as Fat Loss Extreme and Toned in 90 Days for women and men and Ripped in 90 Days for men.
V Shred is based in Las Vegas, Nevada. For more information visit http://www.vshred.com
