SKINCARE 911:
WHAT TO DO WHEN A SKINCARE DISASTER ERUPTS BEFORE A HOLIDAY PARTY
www.drmanishshah.com

It's something every woman fears: Waking up on the day before or day of a big holiday party with an unsightly skincare emergency. How many women have cancelled an invitation because a cold sore erupted? How about cystic acne on your chin that appears New Year’s Eve? While neither of these are "life threatening" conditions, for a woman, they might as well be. There are solutions. Dr. Manish Shah is a Denver Board Certified Plastic Surgeon. As a father to girls, he can relate to these skincare 911s and shares his expertise on what can be done to mitigate various conditions.
Problem: Cold Sore
Solution: Dr. Shah offers cortisone injections to patients who want to look better faster. "Very diluted cortisone into the cold sore, this can bring the inflammation down quite rapidly," he says. If you are afraid of needles, call your doctor and ask him/her to call in a prescription for Valtrex, Famvir, or Acylovir, Dr. Shah says. You can pick up Abreva, an over-the-counter medication. If you can't make it to the pharmacy, you can try some old-fashioned remedies: Visine will help take the red out. You can also use a cold compress and Tylenol or ibuprofen.
Problem: Allergic Reaction
Solution: The first thing you need to do is stop eating or using whatever is causing the allergic reaction. If the reaction happens a few days before an occasion, Dr. Shah recommends using hydrocortisone cream twice a day and taking Allegra, Claritin or Zyrtec which are longer acting and less sedating than Benadryl. Try a whole-milk compress for 10 minutes twice a day. For allergic reactions, use the hydrocortisone cream and then cover up redness by canceling it out entirely. The opposite of red is green, so apply green tinted concealer on the red area. The combination will create a flesh-toned hue. A good quality tinted moisturizer naturally has green/yellow undertones and also provides moisture to dry skin. "If this type of reaction is something you have never experienced before, go immediately to your dermatologist," says Dr. Shah.
Problem: Cystic Acne Breakout
There are some people who use a lancet or small knife to cut into a cyst and fish out the clogged part of the pore. "Cutting open a cyst is extremely risky. You not only run the risk of getting an infection, but you also run the risk of scarring, as in a permanent skin indentation or protrusion," says Dr. Shah. And what if you cut open a cyst but can't squeeze out the root clog? You don't know where the root is or how deep it resides inside your skin. You can't even be 100% confident that you will be able to completely remove the hardened plug of the cyst. If any remnants of the clog remain, the cyst is likely to get re-inflamed and come back even worse. It's also not exactly good to cut open skin and dig around and squeeze the wound. Doing so will only make a bloody mess, increase the chance of skin scarring, and prolong the time it takes the cyst to heal.
Solution: Here's a secret that many a supermodel or actress use: Steroid shot. Dr. Shah explains that, "when we discuss treating acne with cortisone or "steroid" shots, we are referring to the process of gently placing a very dilute quantity of a "glucocorticoid" steroid into the cyst. Glucocorticoids are a class of steroid molecules that are naturally produced by our bodies and have numerous functions including the regulation of human metabolism, immunity, and inflammation. They have very potent anti-inflammatory effects, so they are often used to treat inflammatory diseases in medicine. They can be formulated as creams to treat skin rashes or as pills to treat systemic disease. They can also be injected directly into local areas of inflammation such as in arthritic joints and inflamed acne cysts. Within one or two days of injection into a cyst, the steroid will shrink the inflammation producing relief of pain and almost immediate cosmetic improvement."
Problem: Puffy Eyes
Solution: The key to reducing the puffiness of puffy eyes is having something cool applied to them. "A cool compress or cooled cucumber slices applied for 5 to 10 minutes can constrict blood and lymph vessels," says Dr. Shah. You can also use cool tea bags, which contain tannins that will help reduce swelling. And since puffy eyes can be caused by a high salt diet or alcohol, try to cut out both before an important occasion.
Problem: Sunburn
Solution: Take a cool bath or shower. Set the water to a cool temperature that's just below lukewarm and relax for 10 to 20 minutes. The temperature will ease the pain, and the water will stop your skin from becoming as irritated. Repeat as often as you need to. Avoid using soap, bath oils, or other detergents as you bathe - they'll irritate your skin and possibly make it even worse. If you have blisters forming on your skin, take a bath instead of showering. The pressure from the shower might pop your blisters. When you get out, don't rub your skin dry with a towel. Instead, let yourself air dry, or pat the towel over your skin in small, gentle movements. Apply cold compresses to your skin. If you're not in a situation where you can bathe, or you'd just prefer not to, you can instead apply cold, wet compresses to your skin. Dampen a washcloth or other piece of fabric with cold water and lay it over the affected area for 20 to 30 minutes. Re-wet it as often as you need to. Apply aloe vera to burned skin. Using the pads of your fingers, gently apply the aloe to your sunburn. Don't "rub it in" all the way, like you might with a regular lotion. Leave it a bit goopy and moist on top of the burn - this helps prevent the skin from drying out and becoming more irritated. Reapply as often as necessary. Treat inflammation with cortisone cream (optional). Cortisone creams contain a small dose of steroids that can work to reduce inflammation to your sunburn.
Problem: Too much filler
The Solution: Dr. Shah suggests doing fillers no sooner than 1 month before a big event to allow time for healing and touch ups. One of the reasons Dr. Shah leans toward hyaluronic acid fillers such as Restylane, Juvederm and Perlane is because they are easily reversed with Hyaluronidase. This product dissolves and degrades the Restylane, Juvederm or Perlane so as to reverse the results of the initial injection. It's a great insurance policy when choosing a practitioner. Make sure yours has it at his/her disposal. Most patients find the immediate results of soft tissue filler treatments very satisfying. If an undesirable result occurs, your treatment provider should be able to discuss and carry out all of the treatment options. Removing the effects of a "filler" treatment can be difficult. This is why any filler treatment needs to be done carefully, conservatively and only by very experienced and Board -Certified Specialists. Hyaluronic Acid based dermal fillers have the additional benefit of being partially or completely reversed with time or with the injection of a commercially available enzyme known as Hyaluronidase.
About Dr. Manish Shah
Plastic Surgeon Colorado | Dr. Manish Shah, M.D. | Denver
Manish Shah, M.D., F.A.C.S. was born in Canada and raised in the Washington, D.C. area. He graduated with honors from the University of Pennsylvania, receiving a degree in biomedical engineering. He then completed his medical training at the University of Virginia, earning his Medical Doctorate. During this time he also completed a one-year fellowship in microsurgery research at the New York University School of Medicine / Institute of Reconstructive Plastic Surgery. As a prelude to his plastic surgery training, Dr. Shah completed a rigorous five-year training program in General and Trauma Surgery at Emory University and the Medical College of Georgia. His formal training in Plastic and Reconstructive Surgery was completed at the Univ. of Tennessee College of Medicine – Chattanooga Unit. After completing his plastic surgery training, he moved to New York City when he was selected for the prestigious Aesthetic Surgery Fellowship at Manhattan Eye, Ear, and Throat Hospital. He underwent extensive, advanced training in aesthetic surgery of the face, breasts, and body at the hands of some of the most renowned cosmetic surgeons in the world. This fellowship is widely considered to be the best of its kind in the world. Dr. Shah is one of only a select few plastic surgeons in the country who have undergone formal post-graduate training in aesthetic surgery.
Dr. Shah’s specialties include revision facial aesthetic surgery, rhinoplasty (“nose reshaping”), and aesthetic surgery of the breast (breast augmentation, breast lift, breast reduction). He is, however, well-trained in all areas of aesthetic surgery.
Dr. Shah’s aim is to obtain a natural appearing transformation that complements the real you!
Dr. Shah is a past Clinical Assistant Professor of Surgery at the University of Colorado Health Sciences Center based at Denver Health Medical Center, the Rocky Mountain region’s only academic Level I trauma center. He is a past Chief of Plastic Surgery at Denver Health Medical Center. He also maintains a private practice in Aesthetic and Reconstructive Plastic Surgery on the Dry Creek Medical Center campus (DTC/Denver) and up in the Aspen Valley (Basalt – in the office of MDAesthetics – Tim Kruse, M.D.).
Dr. Shah is a member of the American Society of Plastic Surgeons, the American Society of Aesthetic Plastic Surgery and the International Society of Aesthetic Plastic Surgery.
Dr. Shah is board-certified by the American Board of Plastic Surgery.
Searchlight Pharma Announces Deal That Adds Divigel® To Its Canadian Portfolio
- Searchlight Pharma expands its women's health portfolio by assuming Canadian distribution and promotional activites for Divigel® effective December 7th, 2018
- Divigel® is a prescription transdermal estrogen product approved and available in Canada that competes in the $17.4 million estrogen gel market in Canada
- Vertical Pharmaceuticals, LLC which holds the United States commercial rights has successfully driven Divigel® to be the #1 prescribed estrogen gel
MONTREAL, Nov. 28, 2018 /CNW Telbec/ - Searchlight Pharma Inc. ("Searchlight") is pleased to announce the closing of a transaction whereby Searchlight will take over the Canadian commercial rights to Divigel®. As a result of the transaction, Searchlight will transition and assume distribution and promotional activities in Canada effective December 7th, 2018. Vertical Pharmaceuticals, LLC ("Vertical") who has the market rights in the United States, will work closely with Searchlight to share its market leadership insights as Searchlight prepares to relaunch the brand in Canada. Financial terms of the transaction were not disclosed.
Divigel® (estradiol gel) 0.1% is a prescription, estrogen gel indicated for the treatment of moderate to severe vasomotor symptoms associated with menopause, that is applied once daily to a small area of the upper thigh. The product is supplied in a tiny, single-use sachet pack and is available in three doses of 0.1% estradiol: 0.25, 0.5 and 1.0 g/day, thereby offering convenient and flexible dosing. According to IQVIA CDH data, the Canadian market for prescription hormonal menopause treatments in Canada was valued at $118 million in 2017, and the estrogen gel market alone totaled $17.4 million.1 In the United States, Divigel® is the #1 prescribed estrogen gel.2
"We are pleased to conclude our fourth business development transaction in 2018, and to add an already-approved and marketed product that complements our existing range of products in women's health, including Estragyn™ Vaginal Cream, Mona Lisa® IUDs, OesclimTDS®., Zestica Moisture™ and AmnioSense®blue," said Mark Nawacki, President and CEO of Searchlight." With Divigel® we expand our ability to offer an even wider range of therapeutic solutions that address women's needs in managing menopause, and deepen our commitment to becoming the Canadian leader in women's health. We are also excited to learn from our partners at Vertical, who have demonstrated impressive commercial traction in making Divigel® the #1 prescribed estrogen gel in the US, as we immediately begin preparations to relaunch the brand in Q1 2019."
About Divigel®
Divigel® (estradiol gel) 0.1%, at doses of 0.25, 0.5 and 1.0 g/day, is indicated for the treatment of moderate to severe vasomotor symptoms associated with menopause3. Each gram of Divigel® contains 1 mg of estradiol. According to the Society of Obstetricians and Gynecologists of Canada, 95% of women experience menopause after the age of 45.4 Divigel® is an estrogen gel available by prescription that has been proven to reduce both the frequency and severity of hot flashes and other vasomotor symptoms associated with menopause. Full details regarding the product's clinical and safety evidence can be reviewed in the product monograph that is available online on the Health Canada Drug Product Database.3
About Searchlight Pharma
Searchlight Pharma Inc., headquartered in Montreal, aspires to become a leading Canadian-based specialty healthcare company through best-in class execution of the search, acquisition, commercialization, and focused development of innovative and unique specialty healthcare products that improve life-long human health and wellness. With a core focus on women's health, urogynecology and urology, our team is committed to improving people's lives by bringing the right products to market. Follow us, learn more about what we do, and get to know our product portfolio at www.searchlightpharma.com.
1 According to IQVIA CDH data for the year ended Dec 31st, 2017
2 http://divigel.com/
3 https://pdf.hres.ca/dpd_pm/00023810.PDF
4 https://www.menopauseandu.ca/what-is-menopause/
SOURCE Searchlight Pharma Inc.
Home for the Holidays - How Evolving Dietary Preferences are Changing the Traditional Holiday Meal
TORONTO, Nov. 28, 2018 /CNW/ - Holiday hosts planning this year's festivities may want to take into account the latest research from Dalhousie University. Canadians are changing their perspectives on meat consumption, with 6.4 million already restricting or eliminating meat from their diets. While the millennial and Gen Y demographic make up 63% of those who are Vegan, 42% of Flexitarians - flexible vegetarians, with some meat consumption – are Baby Boomers. Reasons cited for the dietary shift include added health benefits of vegetarianism, environmental concerns, meat prices and animal activism.

While the festive turkey and ham may still be on the menu, ensuring all guests get up from the dinner table satisfied has home chefs exploring new options. Sweet Potato Chronicles' cookbook authors and bloggers Ceri Marsh and Laura Keogh, who focus on "the never-ending story of the well – fed family" have created quick and easy vegetarian options that will appeal to all dinner guests. Marsh and Keogh are conscious of what they are feeding their families but have a realistic approach in juggling busy schedules with healthy meal planning. "There are so many excellent meatless, plant-based options available that whipping up a totally vegetarian menu is easy, delicious and healthy", says Keogh. The duo have developed a vegetarian holiday menu that includes everything from hors d 'oeuvres to main dishes.
"Our cucumber hors d'oeuvres topped with ricotta cheese and Yves Veggie Bacon take literally less than 15 minutes to make", says Marsh. For the main course, consider putting a unique spin on a classic. "Who doesn't love lasagna?" adds Keogh. "We created a sweet potato lasagna that swaps out noodles for sweet potatoes and incorporates Yves Italian Ground Round. These subtle changes to a well-loved dish make it a healthier option, while still satisfying that comfort food craving." Other recipes include veggie Italian sausage and fennel prepared in a sheet pan, Yorkshire pudding bites prepared with veggie ground round, mushrooms and horseradish and zucchini enchiladas. If the crew is craving the next day left over traditional turkey sandwich, the grilled veggie turkey slice, camembert with cranberry sandwich will fit the bill.
"Another added benefit of a vegetarian holiday meal is that the prep time is considerably shorter," says Keogh. "You can spend time with friends and family without spending six hours in the kitchen stressing that the turkey is under cooked or over cooked."
The tilt in eating patterns has staying power with several governments around the world adjusting their food guides. The new Canadian Food Guide encourages an increase in plant-based alternatives. "The explosion of plant-based options at groceries stores and restaurants make it a lot more accessible to live a meatless or reduced meat consumption lifestyle. Yves Veggie Cuisine® is at the forefront of this movement," says Sandro D'Ascanio, Vice President, Marketing and R&D of Yves Veggie Cuisine®.
Yves Veggie Cuisine® products:
- Do not have artificial colours or flavours
- Do not have artificial preservatives
- Do not contains trans fat
- Many have a good source of protein
- Are Non GMO project verified
ABOUT Yves Veggie Cuisine
Yves Veggie Cuisine® is Canada's #1 brand of delicious plant-based protein options. For over 20 years, Yves Veggie Cuisine® has always been committed to creating delicious products that meet the needs of the health-conscious consumer. With over 40 types of plant-based products, Yves Veggie Cuisine® provides nutritious options that can replace ground beef, hamburgers, hot dogs, deli slices and other favourites foods because they do not contain meat and are packed with veggies and nutritious grains. Yves Veggie Cuisine® products do not have artificial colours, flavours, preservatives or trans-fat. They are proudly non-GMO project verified and strive to achieve ultimate customer satisfaction, producing high quality products in an environmentally friendly way.
Images and recipes of Ceri and Laura's creations are available for download on the OverCat Media Hub at http://overcat.com/media-hub/. To request a password for The Media Hub please email cb@overcat.com. Ceri and Laura are available for interviews and cooking demonstrations, please e-mail Gillian DiCesare, gd@overcat.com or Chelsea Brooks, cb@overcat.com for assistance.
SOURCE Yves Veggie Cuisine
NBA player Lauri Markkanen gives up eating red meat to lower his personal carbon footprint

The individual’s role in combating climate change is becoming a prevalent topic, following the release of the IPCC report this autumn. Leading by example, NBA star Lauri Markkanen is taking a stance by changing his daily eating habits. As his first action to combat climate change on a personal level, the Chicago Bulls player has given up eating red meat.
“As my first action for #DontChoke, I pledge to stop consuming red meat as a concrete step towards minimizing my personal carbon footprint. Every move counts, play your part”, Markkanen declares on social media.
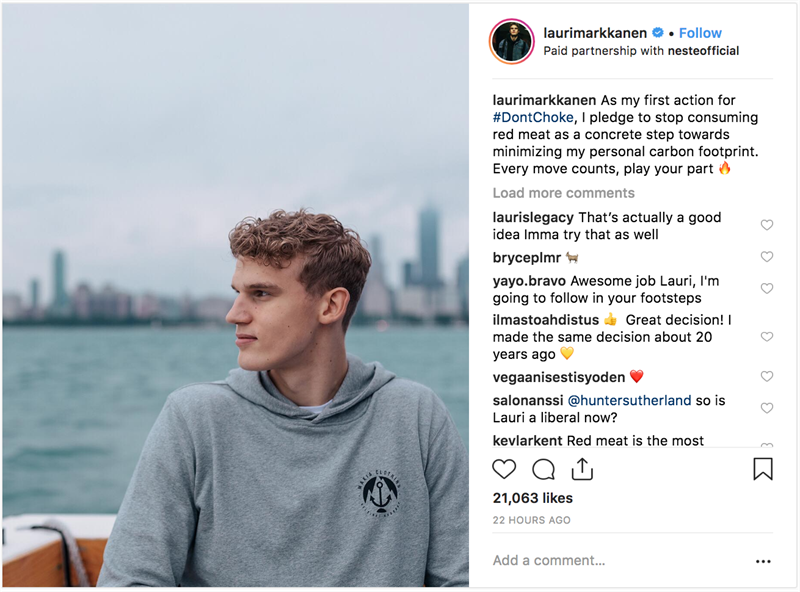
Following his #DontChoke collaboration with renewable energy company Neste, the decision is an exemplary step in doing his part in the fight against global warming.
Markkanen explains, that having recently become a father has made the well-being of the environment even more significant to him. Markkanen encourages his fans to follow in his footsteps to ensure a better environment for generations to come. “I want to do everything in my power so that my child will grow up in a clean environment, like I did. This is a call to all my fans to do their share”, Markkanen explains.
The #DontChoke campaign is a call to action for individuals to do their share in battling climate change. The NBA player kicked off the campaign by shooting hoops with a basketball covered with a hand painted visualization of this year’s heat map. Throughout the on-going campaign, Markkanen will consider the elements of his own lifestyle and opt for more sustainable alternatives. Leading by example, the NBA player hopes to encourage others to make their own climate pledge.
Healthy Lifestyle Habits Can Prevent Dementia
Chattanooga, TN, Nov. 27, 2018 ― Dr. Timothy R. Jennings speaks expertly on a subject that concerns over 5.5 million people across the nation: how to prevent dementia and keep our mind sharp as we age. A psychiatrist and international speaker, Jennings introduces his new book, recently rated #1 by Amazon in books on dementia, The Aging Brain: Proven Steps to Prevent Dementia and Sharpen Your Mind.
Dr. Jennings prescribes simple, everyday actions we can take to stave off disease, promote vitality, and prevent dementia and late-onset Alzheimer's. "The choices we make now can help us to keep our minds sharp and maintain our independence as we age,” says Jennings.
An easy-to-use guide to maintaining brain and body health throughout life, The Aging Brain is based on solid, up-to-date scientific research, and the interventions discussed can prevent progression toward dementia, even in those already showing signs of mild cognitive impairment. The recommendations also may help reduce disability and depression.
"This book isn't just for people hoping to slow the aging process,” says Jennings. "It's also for anyone who is a caregiver to someone at risk of or already beginning to suffer from dementia. It offers a hopeful, healthy way forward.”
Jennings, who maintains a private practice in Chattanooga, TN, has authored several books, including The God-Shaped Brain and The God-Shaped Heart. He is a Distinguished Fellow of the American Psychiatric Association and Fellow of the Southern Psychiatric Association, and is president and founder of Come and Reason Ministries.
For more information about Dr. Jennings, please visit the website: https://www.agingbrainbook.com.
To connect with Dr. Jennings, please visit: https://www.facebook.com/DrTimJennings/ and https://twitter.com/timjenningsmd.
The Aging Brain: Proven Steps to Prevent Dementia and Sharpen Your Mind
Baker Books
Released: June 2018
ISBN-10: 080107522X
ISBN-13: 978-0801075223
###
Reviews for The Aging Brain: Proven Steps to Prevent Dementia and Sharpen Your Mind:
Dr. Caroline Leaf, Cognitive Neuroscientist, Communication Pathologist and Author: "Great advice and excellent science on aging! It's well worth following and applying these principles so as to age the way we are supposed to.”
Rodney A. Poling, MD, DFAPA, medical director, Behavioral Healthcare Center, Columbia TN., and board-certified geriatric psychiatrist: "A well-researched and commonsense book aimed at helping one understand the complexities of dementia, while offering recommendations for maintaining healthy brain function into our later years.”
Michael Lyles, psychiatrist, author, and speaker: "Dr. Jennings clearly describes how to practically manage the medical and lifestyle variables that can positively impact brain health and the process of aging. Age is a number, but getting old is a lifestyle.”
Sunniva Announces Acquisition of California Distribution Company to allow for STATE-WIDE Dissemination of Sunniva branded Cannabis products
- LTYR Logistics, LLC to become Sunniva’s logistics and technology distribution platform to drive sales from Sunniva’s large-scale cannabis production facilities, following the launch of Sunniva branded products commencing Q1 2019
- Sunniva acquires highly skilled management and sales team with a retail network of more than 120 licensed retail dispensaries throughout California
- Compliant distribution is a vital segment of the highly regulated California cannabis industry with track and trace implementation coming in January 2019. For all movement of cannabis products in California, distributors are subject to additional responsibility for product testing and safety as well as state tax collection
VANCOUVER, BC – November 27, 2018 – Sunniva Inc. (“Sunniva” or the “Company”) (CSE: SNN) (OTCQB: SNNVF), today announced the acquisition of 100% of LTYR Logistics, LLC (“LTYR”) (the "Acquisition"), a California-based cannabis distribution company headquartered in San Diego. LTYR’s strong leadership and proven ability to effectively execute growth strategies utilizing its distribution capabilities within the cannabis space are the primary drivers of this acquisition. LYTR will play an instrumental role in driving Sunniva’s leadership position in California as a truly vertically integrated cannabis company across the entire value chain, from seed to sale.
Kevin V. Wilkerson (CEO) leads the LTYR team and has joined Sunniva as the Chief Operating Officer of Sun CA Holdings, Sunniva’s 100% owned California operating subsidiary. Mr. Wilkerson is a Harvard Masters degree graduate and has been a successful entrepreneur in numerous businesses. Kevin has been active in the cannabis space since 2014, providing operations, distribution logistics and technology expertise to the industry. He retired as a Colonel in the US Army after a distinguished 24 year career where he commanded over 4,000 troops and received the Bronze Star. In connection with the acquisition, Mr. Wilkerson will be responsible for all of Sunniva’s California operations.
The other co-founders of LTYR have also joined Sunniva in various management roles. Jay Myers will become Vice President of Sales in California and Brad Neeld will become Vice President of Distribution. Mr. Myers has operated in sales within the cannabis space in both Colorado and California since 2012 following a successful executive management, sales/marketing, and leadership career for companies such as West Coast Consulting and Bank of America. Prior to that, he served 12 years as a Navy SEAL. Mr. Neeld was one of the first large-scale commercial cultivators in Colorado before entering distribution in California. His retail relationships and cultivation knowledge reach far beyond distribution strategies and include scientific aspects of the cannabis core DNA and molecular strain structure.
“Over the last six months, we have evaluated several distribution opportunities to complement our vertical integration strategy with the goal of being able to sell all of the products we produce in our California facilities. Our operations and marketing teams have been working closely with the LTYR team analyzing consumer market data, demand metrics and pricing economics to better define all upcoming Sunniva product lines in preparation of our brand launches commencing in the first quarter of 2019. Kevin and his team are proven distribution, operations and execution specialists and we have the utmost confidence in their distribution capabilities as Sunniva ramps up for large-scale production in 2019 from both of our high-tech greenhouse and extraction facilities,” said Dr. Anthony Holler, CEO of Sunniva. “Over the past quarter, we have been actively manufacturing and stockpiling inventory for our brand launches and we are excited about achieving significant revenue from all our vertical growth opportunities in 2019.”
Kevin Wilkerson, CEO of LTYR Logistics added, "One of the biggest challenges that distribution companies in California are experiencing is getting access to compliant, pesticide-free cannabis products on a large-scale. Compliant cultivation at scale is the quintessential pillar in California for the future success of any brand, distribution company or retail chain. We are very excited to join a preeminent partner like Sunniva that will ensure safe, reproducible, premium-quality cannabis products on a very large scale. After touring both Sunniva’s 325,000 square foot purpose-built cannabis greenhouse and the extraction facility in Cathedral City, we were very impressed with the level of meticulous design and implementation of top tier commercial agriculture technologies and automation. While we had a variety of opportunities available to us, partnering with Sunniva was the most logical choice for our shareholders to take our business to the next level.”
LTYR – Further Information
- Revenue generating and operating in partnership under a third-party distribution license in Northern California
- LTYR to utilize Sunniva’s existing Cathedral City distribution and delivery license
- Sunniva purchasing a 4,200 sq. ft. proposed distribution facility in Long Beach, CA
- Fully automated sorting, weighing, packaging machines for large-scale distribution
- Additional distribution platform for real-time delivery solutions
- LTYR to be compliant with “track and trace” regulations coming January 2019 to California
LTYR – Transaction Details
- Binding term sheet signed for the acquisition of 100% of the equity interests of LTYR
- Consideration: 1,861,971 of Sunniva common shares at CAD $4.26 per share with 620,657 of such shares to be subject to achieving operational milestones
- Expected Closing Date by the end of Q4 2018. Closing is subject to conditions, including negotiation and execution of definitive agreement, receipt of any required approvals, and satisfactory completion of due diligence.
About Sunniva Inc.
Sunniva, through its subsidiaries, is a vertically integrated cannabis company operating in the world’s two largest cannabis markets – Canada and California. Our ability to leverage our large-scale, purpose-built cGMP designed greenhouses, offering better quality assurance with cannabis products free from pesticides, uniquely positions Sunniva as a leading supplier of safe, high quality products at scale. Through our strategically positioned cultivation and extraction facilities, we are launching Sunniva branded products in various product categories including flower, pre-rolls, premium concentrates, vape cartridges, edibles and beverages. We will have inhouse compliant distribution and continue to aggressively pursue retail expansion in the near term in California. Sunniva's management and board of directors have a proven track record for creating significant shareholder value both in the healthcare and biotech industries.
This news release includes statements containing certain "forward-looking information" within the meaning of applicable securities law ("forward-looking statements"), including, but not limited to, statements relating to the proposed benefits of the Acquisition and the plans for Sunniva, and statements with respect to the conditions of completing the Acquisition, including negotiating and executing the definitive agreement and regulatory approvals. Forward-looking statements are frequently characterized by words such as "plan", "continue", "expect", "project", "intend", "believe", "anticipate", "estimate", "may", "will", "potential", "proposed" and other similar words, or statements that certain events or conditions "may" or "will" occur. These statements are only predictions. Various assumptions were used in drawing the conclusions or making the projections contained in the forward-looking statements throughout this news release. Forward-looking statements are based on the opinions and estimates of management at the date the statements are made and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as expressly required by applicable law.
10 Common Mistakes You Might Be Making At The Gym
As people begin to think about New Year’s Resolutions and Fitness Goals, you must remember just paying for a membership doesn’t guarantee the results you want.
www.vshred.com

Many people start their year off buying a gym membership and new gym attire. While the first step to transforming your body is, in fact, deciding to commit to restructuring your lifestyle at the gym and in the kitchen, it is common for people to make mistakes that jeopardize their progress. These mistakes can chip away at their enthusiasm to work out and ultimately result in people giving up on their goals.
“As a trainer, my job consists of helping clients assess where they are and where they want to be. Giving them a clear pathway helps avoid mistakes that often cause people to quit too soon and forfeit their goals,” says Vince Sant, the co-founder and lead trainer of V Shred, the online training platform that has taken the world by storm in recent years.
Vince says these are some of the most common mistakes people make when starting off their fitness journey.
- Failing to Assess Your Body
“Trainers and gym staff see this all the time. People who are new to working out and get a few workouts from a friend and head into the gym without analyzing their bodies with a trainer.” This becomes harmful because these people are not trained to pinpoint their body’s imbalances, where their weaknesses lie and how their body is compensating subconsciously. “They go to the gym pay for the membership and spend months doing exercises incorrectly and damaging their joints and their back.”
- Failing to Set Realistic Goals
Nowadays a lot of people scroll through social media and try and mimic the amount of weight being lifted and want a six pack in two weeks. Contrary to popular belief, fitness models and trainers don’t start off deadlifting 200 lbs. They train for months, maybe years, perfecting their form. When you set major goals with an unrealistic time frame you are setting yourself up for disappointment. “A lot of what a trainer does is manage your goals and give you honest timelines to get you there. We cheerlead you through the rough times and the plateaus,” says Sant.
- Consistency is Key
New gym goers lose their excitement over working out a few weeks in. They start skipping workouts and making excuses. This will add to the time it will take for you to reach your goals and may even hinder your enthusiasm overall. Push yourself through the first few months of working out and eating nutritiously. Make a habit out of working out. Consistency will become easier once you start seeing those desired results
- Delaying Your Workouts Into The Night
We get it. People work tremendously hard and they have errands to run and people to care for. People want to sleep as much as possible. “Here’s a pro tip: If you want consistency to be an easier hurdle to overcome… Plan to workout in the morning. In life, things come up and schedules change. The morning will always be more available than your night. We also tend to make more excuses at night and let things override our plans to work out,” says Sant.
- Giving Up When You Hit A Plateau
If you hit a plateau it means you have consistently been doing the same routine for an extended period of time. Kudos for the consistency, but that is just one part of getting the results you want. “You need to learn or get guidance on how to effectively change your workouts to continue challenging your muscles and prevent your body from falling into a comfort zone with your regular routine,” explains Sant.
- Overdoing the Cardio
Don’t misunderstand, cardio is an important part of any fitness journey especially one that is centered around weight loss. However, in the eagerness of the first few weeks people who start going to the gym concentrate overwhelmingly on cardio and needlessly exhaust their body. Trainers see it all the time, people who spend 2 hours on the elliptical. You’d be better served to do a 30-minute cardio session followed by light weights and circuit training. Remember you want to strengthen the muscle as well.
- Unorganized Lifting
When you are working on weight training you have to educate yourself on what exercises belong in a superset together and how much is wise to lift when shooting for a certain amount of repetitions. “Gym goers often lift too heavy making it difficult to complete a set or too light, failing to challenge the muscle,” says Sant. It is important that you don’t lift too heavy out of vanity and that you don’t too light out of laziness. When doing supersets (two different movements, one after the other you without rest it is important that you learn about the movements that go together as one might affect your form for the other and open you up for injury.
- Record Your Progress
Failing to record the amount of weights and repetitions per set that you can lift per movement easily leads to plateaus and lack of interest. If you don’t record what you are doing then you have no way of increasing intensity meaning you will not get any better. “Working out without a clear goal for each movement can become boring. You lose interest and consciousness about what you are doing. Record what you are doing, perform it consistently and then increase intensity to avoid plateaus.
- Stretch
For many, stretching is common sense. Many people still ignore the importance of it. Working out without stretching can lead to reduced muscle mind connection, poor form and muscle strain. Stretching helps your range of motion and aids in preparing you mentally and physically for the workout ahead.
- Failing to take an Active Rest Day
Many people go all out the first few weeks of their fitness training looking desperately for quick results. This is not wise. Taking a rest day allows your muscles to heal. An active rest day is a day where instead of training at full power you incorporate some sort of low-intensity cardio or HIIT training. This way you keep your body active and don’t let any soreness settle in while still resting from your much more comprehensive workouts.
It is important that before beginning a fitness journey you assess where you are and where you want to be. Seek out help from trainers at your gym or through an individualized online program like V Shred.
About V Shred:
V Shred is the fastest growing fitness and nutrition brand in the world offering online training programs designed to put the fun back into fitness and nutrition. V Shred provides a results-driven enduring lifestyle change instead of a frustrating battle that is easy to give up on. With a support network comprised of trusted accredited advisors and virtual personal trainers, people meet their fitness, nutrition, and goals.
The company, co-founded in 2015 by Nick Daniel, Roger Crandall, Kevin Pearn, and Vince Sant, sought out to create a healthy fitness movement specifically designed to deliver profound changes in your body with the minimal amount of workout time. V Shred has created some of the world's most popular fitness and nutrition programs such as Fat Loss Extreme and Toned in 90 Days for women and men and Ripped in 90 Days for men.
The diet that accompanies the workouts in these comprehensive fitness programs, offers plenty of healthy food options and recipes. V Shred’s supportive coaches encourage “portion empowerment” which inspires people to eat and enjoy food knowing it’s the fuel they need to achieve the results they seek.
V Shred is based in Las Vegas, Nevada. For more information visit http://www.vshred.com
How to KEEP Your New Year’s Fit Resolution
Kickstart your 2019 goal with special retreats designed to keep your commitment
Nov. 27, 2018, Chicago, IL—According to Psychology Today, 71% of people give up their New Year’s Resolution in the first month! Retreats Unlimited, the premiere wellness retreat company in the industry, has curated a host of retreats to help people start and KEEP their 2019 wellness goals. And for Travel Tuesday, you can save $300 off any retreat when booked by Dec. 5, 2018.
Brooke Burke Body Transformation Retreat

Winner and former host of Dancing with the Stars, Brooke Burke brings her wildly popular body transformation retreat to the Don Cesar in sunny Florida Jan. 24-27, 2019. Brooke has an inherent talent of connecting with guests and leading them to mental and physical breakthroughs during the four-day retreat. Her spitfire personality will light a fire under you to keep your resolution.
Mental Fitness Bootcamp

Who doesn’t want to be happier in 2019? During the Jan. 24-26, 2019 Mental Fitness Bootcamp at Rancho Valencia in California, Dr. Zelana Montiminy, a prominent figure in positive psychology will share the secret sauce to happiness (hint: It’s not what you think it is). You’ll leave with a mental fitness toolbox to make 2019 your best year yet.
Fit and Fun with The Freemans

This retreat Jan. 31-Feb. 4, 2019 is the first of our couples retreats! Popular BeachBody fitness trainer Joel Freeman and his personal trainer wife, Breanne, lead couples in a retreat that enhances romantic connection through fitness and fun. With the beautiful CostaBaja Resort & Spa in LaPaz, Mexico as the backdrop, couples will start 2019 with a better connection and a new fitness routine to enjoy together.
A Place of Peace

If your goal for 2019 is to let go and have more peace in your life, the Place of Peace retreat led by Michelle Steinke-Baumgard and Keith Baumgard Jan. 31-Feb. 4, 2019 at CostaBaja Resort in LaPaz, Mexico is for you. Through breath work, meditation and Pilates, you will learn how to release, relax and create peace in the new year.
Live Life Like an Adventure

If you’ve been living small and need a breakthrough to achieve your dreams, you’ll find it at the Live Life Like an Adventure retreat led by Sonia Satra Feb. 7-10, 2019 in sun kissed Scottsdale, Arizona. Focused on indoor and outdoor adventures, Sonia helps you break out of being sucked through radical, profound action.
For more information, images, or to interview any of our influencers, please contact Tammy Petersen at tammy@myretreatsunlimited.com
About Retreats Unlimited
There are many retreats out there, but none that bring together top-notch instructors, unique destinations and experiences, luxury accommodations, and an unparalleled level of service like Retreats Unlimited. Retreats Unlimited is a full-service luxury retreat company offering yoga, fitness, self-improvement, boot camp and wellness vacations. Our company is led by a handpicked team with over two decades of experience in the luxury travel and wellness travel space. www.myretreatsunlimited.com
An innovative new study advances personalized medicine for prostate cancer patients
The first prostate precision medicine trial to use liquid biopsies for genomic testing
KINGSTON, ON, Nov. 27, 2018 /CNW/ - A new clinical trial, opening across Canada, is considered a major advancement in precision medicine for prostate cancer and the first of its kind in the world. The IND.234 clinical trial, conducted by the Canadian Cancer Trials Group (CCTG), uses liquid biopsy technology to screen for genomic markers in prostate cancer patients.
After the liquid biopsy analysis, patients with specific DNA markers are assigned to one of five new therapies targeted at their unique form of prostate cancer. Researchers want to see if the markers identified in the screening process can help predict which patients will be helped the most by the targeted treatments.
"There is an urgent need to find more effective therapies for men with advanced prostate cancer and an individual's cancer is unique, so a one-size-fits-all solution may not be the best," says Dr. Kim Chi, Medical Oncologist, and Medical Director at BC Cancer who is leading the trial. "We want to identify men whose cancers will have the best chance to respond to the experimental new drug therapies we are testing in this trial."
Jim, a trial participant, shares his experience: "When I was first diagnosed with prostate cancer, I understood that this type of cancer was not good. I was offered the chance to receive a new potential treatment and I was willing to try anything that might make a difference. They sent my blood to be tested in BC and then I was enrolled, it was simple. Now I take my new treatment pills and track any side effects."
Although tumour samples taken at diagnosis can be tested for DNA markers, in order to provide current genomic information patients would need an additional invasive biopsy. Using a liquid biopsy to provide the updated information could remove the need for surgery.
"The technology and computation required to study a person's cancer using only a blood sample is very novel and experimental. This team has helped lead the charge for liquid biopsies to be part of prostate cancer clinical research," says Dr. Alexander Wyatt who is leading the DNA analysis and is a Senior Research Scientist at the Vancouver Prostate Centre and the Vancouver Coastal Health Research Institute. "Few other research studies in the world are able to draw upon this combination of advanced prostate cancer focus and liquid biopsy tools."
Canadian research innovation and collaboration - The IND.234 trial is a perfect example of Canadian excellence in research and innovation translating into new treatment approaches. The trail is supported by a core grant from the Canadian Cancer Society, with funding from the BC Cancer Foundation and 3CTN. The Vancouver Prostate Centre performs the biopsy testing supported by Prostate Cancer Canada with additional partners contributing to specific treatment arms of the study.
SOURCE Canadian Cancer Trials Group
One Easy Action to Combat High Cholesterol

Exercise and controlled nutrition are the best and the most effective ways to fight cholesterol. Unfortunately, genetics can dictate the way your body will behave, and you might run into difficulties trying to stay in good condition. It might occur that, no matter how hard you work out, or how healthy your diet is, the level of cholesterol remains high. Fortunately, there’s a solution.
There are a plethora of nutritional supplements out there that are efficient in fighting cholesterol. Some of them are more successful, while others are not as much. We did our research and chose four best supplements to share with you.
- Omega 3 Supplements
Omega 3 fatty acids are healthy fats that are good for your heart, brain, and overall condition. They also lower the risk of heart disease and reduce the level of triglycerides. One of the most excellent benefits is that they help to reduce the level of cholesterol in the blood. Sounds almost too good to be true, right? Sadly, your body can’t create these acids, so you need to get them from your diet. Omega 3 supplements come in capsules, and you can buy them in any pharmacy.
2. Niacin
Niacin is more of a drug than a food supplement and is taken in doses of 1-3 grams a day. The way it helps is by lowering LDL, or, so-called, “bad cholesterol” while boosting HDL, or “good cholesterol” at the same time. If you thought that there’s some magic to it, there isn’t. This medication is nothing more than a B vitamin and available at pharmacies. Niacin is often prescribed primarily for people with low HDL and those with elevated triglycerides.
3. Cholesterade
Invented by the creator of Gatorade, Dr. J. Robert Cade, this product lowers cholesterol levels in as little as eight weeks. It reduces LDL and triglycerides and raises HDL. At the same time, Cholesterade® is gluten-free, GMO-free, and is suitable for vegans and it tastes great. You can choose among three fruit flavors, mix it with water and drink it twice a day.
4. Red Yeast Rice Extract
For hundreds of years, red rice has been a heart remedy in China. It wasn’t until recently that the western scientists derived its extract by fermenting red rice and turned it into a supplement. Its key component is monacolin K, a compound that lowers cholesterol production in the liver. The right dosage can vary depending on the process of fermentation used, so if you decide to use red yeast extract, be careful and ask for your doctor’s advice.
To sum it up, problems with cholesterol can be severe, so it would be better to prevent them than fight them when they appear. Sometimes it is necessary to take medications or nutrition supplements to secure good health and a happy future. But remember: no matter how determined or informed you are, don’t forget to consult your doctor before taking any of the products. And if you want to learn more about Cholesterade, please visit cholesterade.com.












