BlueWind Medical Appoints New Executive Chairman
Daniel Lemaitre brings more than three decades of medical device experience
HERZLIYA, Israel, Nov. 19, 2018 /CNW/ - BlueWind Medical, developer of a miniature, wireless neurostimulation platform for the treatment of multiple clinical indications, is pleased to announce that Daniel T. Lemaitre will assume the role of Executive Chairman.
"Dan is a savvy and seasoned medical device executive. His operational and financial acumen make him the ideal person to lead BlueWind's efforts to garner market clearance and to commercialize BlueWind's neuromodulation therapy platform. Dan will be an outstanding addition to the company's executive team," said Efi Cohen Arazi, BlueWind board member and the CEO of Rainbow Medical, an operational investment company that founded BlueWind in 2010.
Lemaitre's C-suite experience includes CEO roles at CoreValve and Direct Flow Medical. He has also served as Senior Vice President of Strategy and Business Development at Medtronic, and held the role of Chairman of Bioventus after it was spun-out of Smith & Nephew in a private equity transaction. He currently serves as Chairman of Endologix (ELGX) and is on the board of Globus Medical (GMED).
Dan earned his financial stripes during his 28 years as a top-ranked medical device Wall Street analyst with Cowen & Company and later with Merrill Lynch.
"I am thrilled to be working alongside CEO, Guri Oron, and the rest of the highly engaged and competent BlueWind team," Lemaitre said. "BlueWind's less invasive technology has the potential to revolutionize the treatment of myriad afflictions, with patient-friendly products that offer clinical efficacy and superior economics. We are initially targeting Over Active Bladder (OAB) a market with large unmet patient needs. A pivotal study to garner FDA approval will commence in 2019."
About BlueWind Medical
BlueWind Medical was founded in 2010 by Rainbow Medical. The company is developing a platform technology of miniature, wireless, neurostimulators that can be injected or implanted in a minimally invasive procedure to treat multiple indications. By putting patients' needs first, BlueWind Medical's team of experienced and dedicated engineers and researchers are creating a versatile and effective platform that will transform neuromodulation as we know it.
About Rainbow Medical
Rainbow Medical (www.rainbowmd.com ) is a unique private operational investment company that seeds and grows start-up companies developing breakthrough medical devices, addressing significant unmet market needs in a diverse range of medical fields.
SOURCE BlueWind Medical
Ontario's health care system under increased strain
TORONTO, Nov. 16, 2018 /CNW/ - Many parts of Ontario's health care system are under increased strain, according to Measuring Up 2018, Health Quality Ontario's 12th yearly report on the performance of the province's health system.
"One of the things we're seeing is hospital overcrowding and how it's both a symptom and a source of cascading pressures throughout the system," says Anna Greenberg, interim president and CEO of Health Quality Ontario.
The report's key findings emphasize the backlog in different parts of the health system, underscoring the hospital overcrowding issue. Some examples include:
- Emergency department visits are on the rise, especially among patients with serious conditions. Plus, patients are spending more time in the emergency department before being admitted to hospital.
- Hospital emergency departments are also facing an increased challenge dealing with the opioid crisis.
- The number of hospital beds occupied by patients waiting to receive care elsewhere continues to increase and is equivalent to more than 10 large, 400-bed hospitals filled to capacity every day.
- Patients are waiting longer to access long-term care, assisted living and home care, from hospital and from the community too.
Despite these challenges, the report also shows bright spots scattered throughout the health system:
- People in Ontario are living longer and less likely to die before the age of 75.
- More people are having cancer-related or general surgeries within the recommended wait times.
- Rates of hospital-acquired C. difficile, a potentially life-threatening infection, continue to improve.
- Fewer children and youths are having their first care for a mental health condition occur in the emergency department.
- More Ontarians are receiving palliative care in their homes in their last days of life.
"The improvements we have seen in areas like wait times for cancer-related or general surgeries are a testament to focused and sustained efforts based on meaningful data by those on the frontlines," says Ms. Greenberg. "With the same system-wide focus that is informed by data, and ingenuity on the frontlines and at regional levels, tackling a complex problem like hospital overcrowding is also possible."
"In order to better meet patient needs, it is essential to understand the conditions and pressures in emergency rooms today, and the profound difficulty our health care system has always faced in creating better ways to care for people, especially in their home and in long-term care," says Anthony Dale, president and CEO of the Ontario Hospital Association. "Accurately measuring and understanding these challenges and their root causes creates the foundation for creating the long-term solutions needed to end hallway health care in Ontario."
Quick facts
Findings in areas that need further improvement
- Visits to Ontario's emergency departments increased by 11.3% over the last six years to 5.9 million in 2017/18, from 5.3 million in 2011/12. Visits by high-acuity patients – those who have more serious conditions – also rose by 26% to 4.1 million from 3.3 million.
- The average time spent in emergency by patients admitted to the hospital from emergency increased to 16 hours in 2017/18, from 15.3 hours in 2016/17.
- Visits to the emergency department for opioid poisoning more than tripled to 54.6 per 100,000 population in 2017, from 15.2 per 100,000 in 2003.
- In 2016/17, an average of 4,233 Ontario hospital beds were occupied every day by patients waiting to receive care somewhere else – such as in a long-term care home or rehabilitation facility. That was the equivalent of more than 10 large, 400-bed hospitals filled to capacity each day.
- The median amount of time people waited from hospital to move into a long-term care home was 31.4% longer in 2016/17 (92 days) than in 2015/16 (70 days). The median wait time for people waiting to move into a long-term care home from the community also increased by 12.9%, to 149 days in 2016/17, compared to 132 days in 2015/16.
- In 2017, fewer patients (32.3%) reported being able to see a specialist less than 30 days after being referred to one, compared to 37.8% in 2016.
- Fewer patients had their surgery within target times in 2017/18 for common surgeries like knee (73.7%) and hip replacements (78.0%).
- More than a quarter (26.1%) of home care clients who received care for more than two months had a primary family or friend caregiver who experienced continued distress, anger or depression in the first half of 2017/18, up from 23.6% the previous year.
Findings in areas that are doing well or improving
- Ontarians are less likely to die before the age of 75. They are losing fewer years of life to premature death, decreasing to 4,188 years per 100,000 people in 2015, compared to 4,897 in 2005. Ontario has one of the lowest rates of potential years of life lost among Canadian provinces.
- The percentage of patients who had cancer surgery within target times for their assigned priority level increased to 87.3% in 2017/18, up from 70.9% in 2008/09. Among patients who had general surgery in 2017/18, wait time targets were also met for 95% of patients, an increase from 90.6% in 2008/09.
- The rate of hospital-acquired C. difficile infections has decreased steadily in Ontario in recent years to 0.22 cases per 1,000 inpatient days in 2017/18, compared to 0.35 in 2011/12.
- Fewer children and youths up to 24 years of age received their first care for a mental health condition in the emergency department. The rate decreased to about 4 in 10 in 2016, which was an improvement from 10 years earlier when the rate was about 5 in 10.
- More than a quarter (25.5%) of people who lived in the community during their last 30 days of life received a palliative-specific home care service in 2016/17, compared to 22.6% in 2011/12.
Measuring Up 2018 also features stories from health care professionals, patients and caregivers to provide a human viewpoint on the performance data.
To read the full Measuring Up 2018 report, visit: https://www.hqontario.ca/Measuring-Up
About Measuring Up
Measuring Up 2018 is Health Quality Ontario's 12th yearly report to Ontarians on health system performance. The data are grounded in a focused set of indicators selected by Health Quality Ontario, in partnership with health experts and system partners. This year's report highlights findings from 47 indicators.
About Health Quality Ontario
Health Quality Ontario is the provincial lead on the quality of health care. We help nurses, doctors and other health care professionals working hard on the frontlines be more effective in what they do – by providing objective advice and data, and by supporting them and government in improving health care for the people of Ontario. Visit www.hqontario.ca for more information.
SOURCE Health Quality Ontario
Clean Eating: How to Maximize Your Fitness and Workout Benefits

Whether you’re looking to lose weight, bulk up or maintain your current weight, it can be an understandably challenging journey. When most people don’t see their results within a specific time frame, it’s easy to become discouraged and throw in the towel. However, it’s always important to remember that the body you’re looking for is found in the kitchen. While fitness plays a role in the journey, your diet is what will make the major impact. In order to see a transformation in your ability to maximize your fitness and workout benefits, consider these four tips for clean eating.
Drink Lots of Water
Water is essential to a fitness journey. When you’re working out, your body loses water in the form of sweat. It also needs water to remain hydrated. Hydration is essential for the person who needs the energy to get through a workout. When a person drinks enough water on a daily basis, they increase their chances of staying fuller for a longer period of time. Do your best to consume the correct amount of ounces based on your body’s needs.
Incorporate Lots of Raw Fruits and Vegetables
Instead of steaming broccoli or roasting the asparagus, look for a lot of raw options first. When you consume fruits and vegetables in their natural state, your body is able to receive all of the vitamins and nutrients in the purest form. Enjoy a bowl of berries with your morning bowl of cereal. Eat a side salad a part of your lunch. Enjoy cucumbers, carrots and celery as a snack with hummus. If you love sushi, try avocado sushi with bits of cucumber, carrots and sprouts for dinner. Get creative with the way you consume raw foods.
Track Your Fitness
When a person is longing to lose weight, it’s always best to look at the number of calories first. If you need to stay within a certain number of calories, use a calorie counter to help you get a better idea of how much you consume on a daily basis. There are companies that do nutritional profilingand can give you a better understanding of the proper consumption of calories you’ll need to achieve your goals.
Drink a Meal Replacement Smoothie
Most people love a delicious drink. A meal replacement smoothie is awesome because it’s filling, delicious and nutritionally-rich. Along with your choice of liquid (water and almond milk are great choices), add vegetables, fruits, and seeds. You can even enjoy a smoothie on the go.
The key is consistency. It’s never easy to get started on this type of journey. However, if you take it one day at a time, you’ll be able to see results in the long run. Plus, you don’t want quick results. When you lose weight quickly, you’ll tend to gain it back quickly. If you’re committed to the long haul, you’ll be able to maintain the goals you’re working hard to achieve.
How Smart Gym-Goers Keep Themselves Safe from Injury

Going to the gym and working out frequently can be terrific for your mood, well-being and health. It can sometimes lead to unintentional physical injuries, however. If you’re an avid exercise enthusiast who wishes to safeguard yourself from injuries, stress and inconveniences in general, these techniques can get you on a track that makes complete sense.
Do Your Warmup Stretches
Warmups are of the essence for people who want to safeguard themselves from unpleasant injuries during workout sessions. Stretching your body for a couple of minutes can help prepare it for all that you have coming for it. If you want to get your physique limber and ready for action, in-depth warmups can be useful. They can often keep injuries away, too.
Refrain from Going Overboard
Working out is good for you. Overdoing it, however, is never a good idea. If you want to protect yourself from physical trauma, then you should refrain from going overboard. Just say no to exercise any time you feel overly exhausted. It isn’t worth the risk. Moderation is key for everything in this world. Workout sessions are certainly no exception to this golden rule.
Take Breaks
Bodies require recovery time. People who want to safeguard themselves from injuries often give themselves regular exercise breaks. Try to give yourself weekends off if at all possible. Some time away from the gym can help your body heal. It can help you come back to your workouts stronger and more motivated than ever before as well. Nothing matters more than giving your body the chance to rest and recuperate regularly. Bodies aren’t unstoppable machines for anyone.
Understand All of the Risks
Stay away from workout equipment and devices at the gym that seem risky or dangerous in any way. If you see an elliptical trainer or a treadmill that doesn’t appear to be functioning correctly, avoid it. Don’t make yourself vulnerable to injuries that are brought on by faulty equipment. Talk to personal injury lawyerswho can explain to you all of the hazards that are possible in workout settings as well. The last thing you want is to break a bone during a routine jogging session, after all.
Moderation is essential for everything in this life. Prudence is, too. If you’re an intelligent workout buff, you need to prepare for all kinds of undesirable scenarios. It’s critical to remember that gym injuries can happen at all times.
University Orthopedics Partners with Boston Children’s Hospital on ACL Repair Trial
Procedure Utilizes Bridge-Enhanced® ACL Repair (BEAR®) Technique to Treat Patients with ACL Injuries
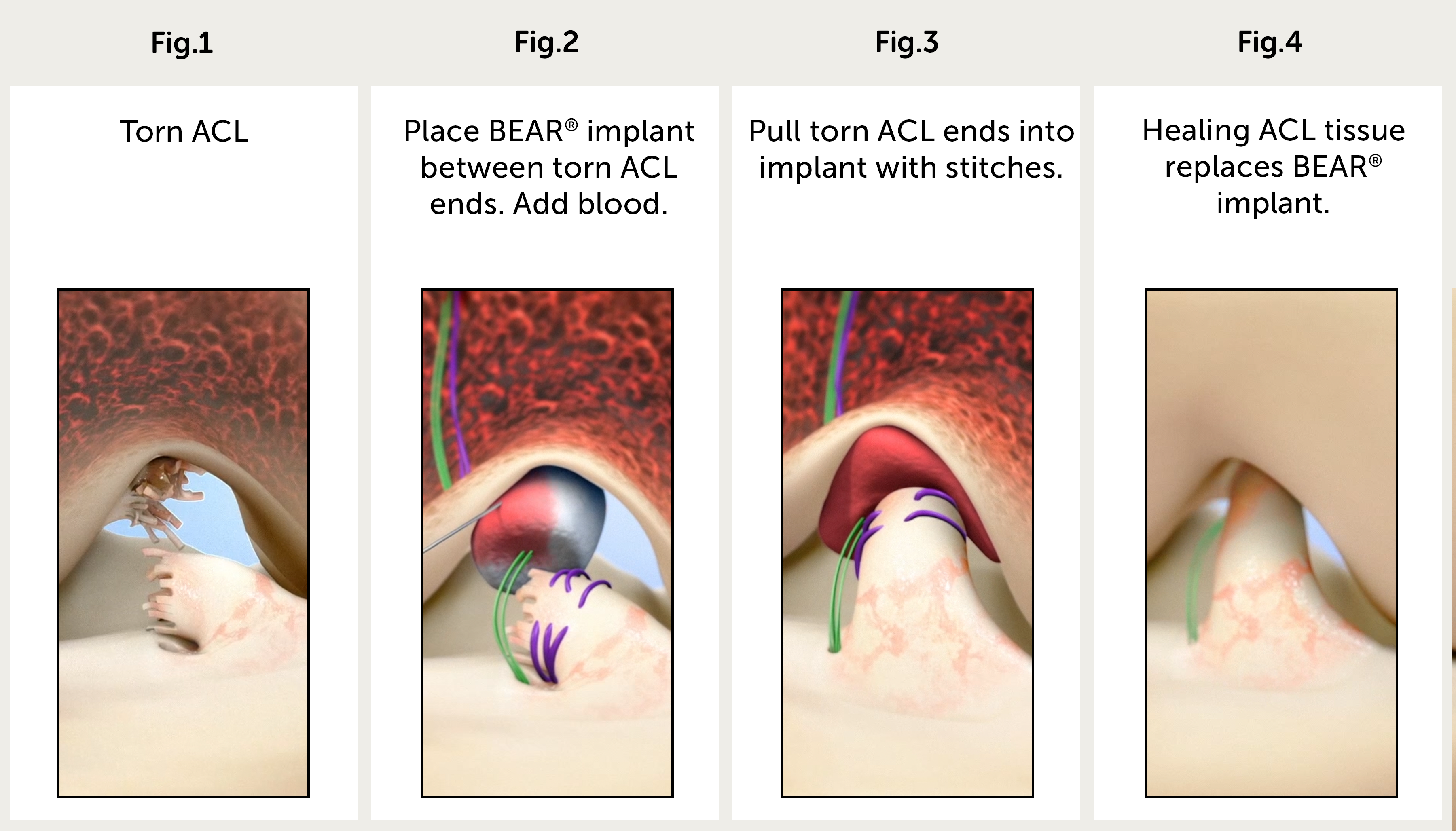 PROVIDENCE, R.I. (November 15, 2018) – University Orthopedics announced that their team of sports medicine surgeons, Drs. Hulstyn, Fadale and Owens, have begun performing ACL repairs with BEAR, a procedure that has been developed by Dr. Martha Murray and her team at Boston Children’s Hospital, with the help of Dr. Braden Fleming and his team at Rhode Island Hospital.
PROVIDENCE, R.I. (November 15, 2018) – University Orthopedics announced that their team of sports medicine surgeons, Drs. Hulstyn, Fadale and Owens, have begun performing ACL repairs with BEAR, a procedure that has been developed by Dr. Martha Murray and her team at Boston Children’s Hospital, with the help of Dr. Braden Fleming and his team at Rhode Island Hospital.
Every year, approximately 400,000 anterior cruciate ligament (ACL) injuries occur. Unlike other sprained ligaments, the ends of a torn ACL do not reconnect and heal on their own. ACL reconstruction is one of the most common orthopedic procedures of the United States and is the standard treatment for the torn anterior cruciate ligament. During an ACL reconstruction, an orthopedic surgeon removes the ends of the torn ACL and replaces them with a graft, usually with patellar or hamstring tendons from the patient’s knee. The new technique, bridge enhanced ACL repair (BEAR), uses stitches in a bridging scaffold (a protein sponge injected with the patient’s blood) to stimulate healing of the torn ACL.
University Orthopedics’ Dr. Michael Hulstyn was the first to perform the surgery at Rhode Island Hospital. “Anterior cruciate ligament reconstruction is the standard of care for a torn ACL with high patient satisfaction and outcomes, but carries the long-term risk of graft failure and knee post traumatic degenerative arthritis. The BEAR procedure allows reattachment of the native ligament and is less invasive than reconstruction surgery. The goal is for a faster recovery time and return of knee stability with high patient satisfaction, and hopefully less chance of arthritis 15 to 20 years down the road.”
Dr. Murray states, “We are now in our third clinical trial and we feel that University Orthopedics and Rhode Island Hospital are a perfect fit to continue this research. Doctors Hulstyn, Fadale and Owens have extensive experience in ACL surgery and we are excited to have them join this study.”
The goal of the current study is to analyze the BEAR procedure and more patients to determine if patient age contributes to the success of the procedure. Up to 250 patients will be enrolled at University Orthopaedics /Rhode Island Hospital and at Boston Children’s Hospital.“So far the results have been very promising. We are thrilled to be part of this exciting trial and appreciate Dr. Murray and her staff for allowing University Orthopedics to continue this groundbreaking work,” says Hulstyn.
About University Orthopedics
University Orthopedics, with clinic locations in Providence, Middletown, East Greenwich and Barrington, is a regional Center for Orthopedics, Sports Medicine and Rehabilitation, with specialties in back and neck pain, joint pain, sports medicine problems, shoulder conditions, pediatric orthopedics, musculoskeletal tumors, hand and wrist problems, hip and knee conditions, trauma, and foot and ankle injuries. UOI includes more than 45 board-certified orthopedic, fellowship trained musculoskeletal and sports medicine physicians. These specialists are faculty members of the Department of Orthopaedics at the Warren Alpert Medical School of Brown University who teach medical students, orthopedic residents, and fellowship sub-specialty surgeons. University Orthopedics leads the way with Basic and Clinical orthopedic research on the latest advances in orthopedic surgery and injury prevention.
About BEAR Clinical Trial
For those looking to schedule an appointment with a BEAR trial physician, please email BEAR.TRIAL@LIFESPAN.ORG or call 1-401-649-1906.
Strainprint™ Technologies, Lumir Lab and Gynica Announce Clinical Trial Partnership
Establishing World’s First and Largest Database of Medical Cannabis Effects on Women
International Study Monitoring Real-time Cannabis Treatment for the Symptoms of Endometriosis Will Leverage Strainprint's Comprehensive Research Platform
TORONTO, Nov. 15, 2018 /CNW/ - Strainprint™ Technologies Ltd., the leader in cannabis data and analytics, today announced a partnership with Israeli research leaders, Lumir Lab and Gynica to conduct the world's first international clinical study on the use of cannabis to treat endometriosis. Endometriosis, a condition where tissue from the uterine lining migrates to other organs inside the body affects roughly 180 million women worldwide. It is estimated that 1 in 10 women between the ages of 15 to 49 will be affected by symptoms of endometriosis during their lifetime.
Strainprint's VP of Research, Michelle Arbus, will work closely with world-renowned cannabis scientist, Professor Lumir Hanus, along with Gynica's Professor Moshe Hod, President of the European Association of Perinatal Medicine and Professor of Obstetrics and Gynecology at Tel Aviv University medical school. The intention of the study is to develop clinically-validated cannabinoid-based products that can be approved for international distribution. Gynica holds a federal license by the Israeli Ministry of Health to research the effects of cannabinoids on women's health and gynecological disorders.
"Endometriosis remains one of the most misdiagnosed and least understood medical conditions, and currently, there is no cure," said Professor Hod. "Strainprint's early observational studies show that medical cannabis treatment has a positive effect on symptoms related to endometriosis, but much more research is required. Our objective is to identify which active cannabinoids, terpenes, and flavonoids, in relative combination, provide the most effective relief, reduce pain and prevent recurrence."
Simultaneous studies in both Israel and Canada will leverage Strainprint's research-ready platform to provide ethics approval, validated questionnaires, custom surveys, real-time treatment tracking and data visualization in support of in-clinic visits. Gynica Senior scientist, Dr. Sari Prutchi Sagiv, will develop the joint-study protocol to be used for clinical trial applications in both countries, based on a common product formulation. Drawing first from Strainprint's 900,000+ real-time patient outcomes and 40 million medical cannabis data points, Lumir Lab will narrow and validate a formulation to focus on at the study. Together, the parties will use Strainprint's recently launched community portal to recruit up to 1,000 patients in each country.
Dr. Prutchi Sagiv says "The combination of Strainprint's big data analytics combined with Gynica's scientific team and clinical research capabilities create a unique and innovative approach for providing evidence-based products to patients worldwide, and moreover for women who are under-treated by current solutions."
Gynica, Lumir Lab and Strainprint expect the clinical study to run on Q2 of 2019.
"It's truly amazing for all of us at Strainprint to get to work with this team of world-renowned scientists and doctors on such a significant medical issue," said Strainprint CEO, Andrew Muroff. "We're committed to helping improve the lives of millions of women suffering from endometriosis."
About Strainprint™
Founded in Toronto in 2016, StrainprintTM Technologies Ltd. is the leading demand-side cannabis data and analytics company. With the world's largest longitudinal, observational data-set of its kind and a mission to advance the scientific understanding of cannabis and its legitimization as a mainstream therapy, Strainprint helps medical cannabis patients and doctors to use cannabis in the most effective and responsible way possible. StrainprintTM data platform supports global cannabis research and provides advanced business intelligence and treatment guidance to producers, retailers, medical practitioners, pharmacies, government and industry. Strainprint is HIPAA, PIPEDA and PHIPA privacy compliant, military-grade encrypted and all patient data is completely anonymized and at rest in Canada. Strainprint can be seamlessly embedded or integrated with most electronic medical records (EMR) and seed2sale software systems. Strainprint Analytics is accessed by customer subscription. The Strainprint App is free to patients and can be downloaded from both the iOS App Store and GooglePlay Store. www.strainprintanalytics.com. facebook, twitter, linkedin.
About Gynica.
Gynica focuses on clinically proven cannabis-based solutions in the field of women's health, developing optimal therapeutic results based on innovative technology and understanding of the pharmacological effects of different cannabinoids and compounds, targeted to specific female-related diseases. The team is led by the President of the European Association of Perinatal Medicine, Professor Moshe Hod, who is also a Tel Aviv University medical school professor of obstetrics and gynecology. Gynica is owned by Asana Bio Group, an Israeli holding company specializing in scientific advancements in the field of medical cannabis.
About Lumir Lab.
Lumir Lab is a research & development lab focused on cannabinoids, led by Prof. Lumír Ondřej Hanuš, a world-renowned analytical chemist and a leading figure in the field of cannabis research, who is responsible for some of the greatest discoveries in the field of cannabis. During his career of 49 years as a cannabis researcher, he isolated and identified the first known endocannabinoid in the human brain, Anandamide. Lumir Lab has established a world-leading position in the development of cannabinoid-based therapeutics through analytical methods and clinical validation, offering a wide range of solutions for companies operating in the medical cannabis industry. Lumir Lab is owned by Asana Bio Group, an Israeli holding company specializing in scientific advancements in the field of medical cannabis.
View original content to download multimedia:
SOURCE Strainprint Technologies Ltd.
For further information: Media Contact: Jessica Moran, 519-494-5379, Strainprint Technologies Ltd., jessica.moran@strainprint.ca
Organization Profile
Strainprint Technologies Ltd.
More on this organization
Non-small cell lung cancer market projected to be worth $14.6bn by 2024, says GlobalData
Following the recent spate of approvals in both small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), the treatment landscape is becoming increasingly difficult to navigate for both physicians and patients, highlighting the need for better education, access, and infrastructure to support physicians’ and patients’ choices of the optimal therapies, says GlobalData, a leading data and analytics company.
GlobalData expects the global NSCLC market to be worth $14.6bn by 2024, over six-fold more than the SCLC market, which is expected to reach only $2.3bn in the same timeframe.
Francesca Blum, MSci, UK Oncology Director at GlobalData, comments, “As SCLC and NSCLC both have more than twenty immunotherapy products currently in Phase II or III development, treatment paradigms for both forms of the disease will become increasingly complex, requiring treatments to be chosen from a growing selection of similar options.”
The FDA has already approved several drugs for lung cancer during H2 2018, including targeted tyrosine kinase inhibitors and immunotherapies. Pfizer has had a particularly good streak, with approvals for Vizimpro (dacomitinib) in September for untreated patients with epidermal growth factor receptor (EGFR)-mutated NSCLC and Lorbrena (lorlatinib) in early November for previously-treated patients with anaplastic lymphoma kinase (ALK)-mutated NSCLC.
Blum continues, “These approvals will boost Pfizer’s lung cancer revenues, which have suffered recently as its original ALK inhibitor Xalkori (crizotinib) has faced sales pressure from Takeda’s and Novartis’ newer ALK-targeted competitors, Alunbrig (brigatinib) and Zykadia (ceritinib), respectively.”
Alongside the much-needed increase of targeted treatment options for NSCLC patients with ALK or EGFR mutations, the use of immuno-oncology is also expanding, particularly into subtypes of lung cancer with significant unmet needs. In August, Bristol-Myers Squibb’s Opdivo (nivolumab) was granted accelerated approval by the FDA for metastatic SCLC, making it the first immunotherapy for this type of lung cancer.
A key aim of this year’s Lung Cancer Awareness Month campaign is to ensure public awareness about screening programs and resources to help with the early detection of lung cancer.
Blum adds, “Improvements in diagnosis will no doubt contribute to increasing global incidence rates, which we currently expect to rise in SCLC and NSCLC at Annual Growth Rates of 2.05% and 3.24%, respectively, giving players further opportunities to gain from this buoyant market.”
GeneNews Announces Q3 2018 Results and Provides Progress Update
Q3 Revenue up 81% year over year
- Leveraged the financings from the Unit Private Placement and the Lind Convertible Security to significantly accelerate promotional activities
- Expanded outreach to enrolled and active practices to accelerate growth. New practices awaiting full on-boarding increasing steadily.
- Conducted early cancer testing for colorectal, lung, prostate and breast cancers in two High Risk Employee groups; preparing for testing of further groups now.
- Approximately 1600 tests processed during the quarter.
- Direct to Consumer testing continues to be built-out, with new initiatives tied to launch of new website and soon a dedicated consumer site.
- Implementation continues within several large healthcare systems with support of LifeX.
- Rebranding of company into single entity behind proven Sentinel Principle technology and advancement of Aristotle: early detection of ten cancers from a single blood sample - a $2 billion opportunity.
- Subsequent to the Quarter: Test volume in October up 30% over previous months.
TORONTO, Nov. 15, 2018 /CNW/ - GeneNews Limited (TSX:GEN) ("GeneNews" or the "Company") today announced operational and financial results for the three-month and nine-month periods ended September 30, 2018 and provided a progress update on its business.
"Billing revenue is up markedly (+81%) from a year ago when we began the process of switching billing companies, and very significantly from last quarter (654%) as the new process gains traction," commented James R Howard-Tripp, GeneNews' Chairman and CEO. "Q3 tests started aggressively, with test volume up 40% during July, but the full quarter was impacted by the hurricanes that affected the South and South East of the U.S., our larger customer states. Test volume in October is again showing growth, with testing of High-Risk employees contributing."
Howard-Tripp further commented, "GeneNews and LifeX this morning announced a partnership which has two specific near-term objectives: the first is to expand the adoption and use of GeneNews' 'liquid biopsy', for the early diagnosis of cancer into large, multi-entity healthcare systems; the second objective is to fully develop and market GeneNews' Aristotle platform, specifically, the first-in-class early detection of ten cancers from a single sample of blood. It is GeneNews' and LifeX's intent to have a transformational effect on early cancer screening and diagnosis."
Q3 2018 Financial Results
All amounts are expressed in U.S. dollars unless otherwise stated and results are reported in accordance with International Financial Reporting Standards.
For the three-month period ended September 30, 2018, we reported a consolidated net loss of $0.7 million, or $0.01 loss per common share, as compared with a consolidated net loss of $0.6 million, or $0.01 loss per common share, for the three-month period ended September 30, 2017. The $0.07 million increased loss results from the $0.07 million impact of the revaluation of warrants, $0.02 change in fair value of conversion liability, the $0.01 increase in finance costs, the $0.1 million increase in cost of goods sold, offset by the $0.04 million increase in revenue and $0.1 million reduction in general and administrative costs.
For the nine-month period ended September 30, 2018, we reported a consolidated net loss of $3.9 million, or $0.03 loss per common share, as compared with a consolidated net loss of $2.6 million, or $0.04 loss per common share, for the nine-month period ended September 30, 2017. The $1.3 million increased loss results from the $0.7 million impact of the revaluation of warrants, $0.6 million change in fair value of conversion liability, the $0.6 million increase in finance costs and the $0.2 million decline in test revenue, offset by $0.6 million lower general and administrative expenses and $0.2 million lower cost of goods sold.
During the third quarter of 2018, we leveraged the significant financing initiatives in Q2, 2018 to support our Four Primary Growth Area initiatives including: small independent practices, high-risk populations/employers, telemedicine and large healthcare systems. JTS continues to assist with the new billing process at IDL which has improved our cash collections on a quarter-over-quarter basis. Revenue from tests was $98 thousand this quarter compared to $54 thousand in Q3 2017 and the $13 thousand in Q2, 2018. Invoicing for tests run but not billed during our transition to the new billing process continues to be submitted to payers and followed up with patients, and we expect collections on these amounts to continue to be collected over the next few quarters.
The Company's financial statements and management's discussion and analysis are available on www.sedar.com.
About GeneNews
GeneNews, an innovator in the liquid biopsy space, is committed to becoming a leader in advanced diagnostics and personalized medicine, serving as a strong commercialization outlet for early detection of cancer and other chronic diseases. Our mission is to identify, assess and make commercially available a comprehensive menu of diagnostics that provide physicians and patients with personalized clinical intelligence and actionable information to improve health out-comes through the early diagnosis of disease. Our Richmond, Virginia-based Innovative Diagnostic Laboratory clinical reference lab specializes in traditional and advanced clinical evidence-based blood testing that helps find, understand, and address cancer risk in patient populations. Currently, IDL offers risk assessment blood tests for four prevalent cancer types - colon, lung, prostate and breast. GeneNews' common shares trade on the Toronto Stock Exchange under the symbol 'GEN'. More information on GeneNews and IDL can be found at www.GeneNews.com and www.myinnovativelab.com, respectively.
Forward-Looking Statements
This press release contains forward-looking statements identified by words such as "expects", "will" and similar expressions, which reflect the Company's current expectations regarding future events. The forward-looking statements involve risks and uncertainties that could cause the Company's actual events to differ materially from those projected herein. Investors should consult the Company's ongoing quarterly filings and annual reports for additional information on risks and uncertainties relating to these forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. The Company disclaims any obligation to update these forward-looking statements, except as required by law.
SOURCE GeneNews Limited
Body and Mind Inc. Strengthens Management Team With Veteran Cannabis Industry Appointment
VANCOUVER, Nov. 15, 2018 /CNW/ - Body and Mind Inc. (CSE: BAMM) (US OTC: BMMJ) (the "Company" or "BaM") is pleased to announce the appointment of Mr. Trip Hoffman to the position of Chief Operating Officer of Body and Mind Inc. as the Company continues expansion and assesses additional accretive opportunities.
"Trip brings significant operational, financial, agricultural and cannabis experience which will be invaluable as we grow Body and Mind to the next level," stated Robert Hasman, Director at Body and Mind. "Trip's experience running Colorado cultivation facilities and dispensary operations will immediately benefit our operations as we expand the premium Body and Mind brand."
Mr. Hoffman is currently the co-owner of a Colorado licensed marijuana cultivation facility and was previously the CEO of a Colorado licensed cultivation and dispensary company. As an operations-efficiency specialist in the cannabis space, Mr. Hoffman has significantly improved the bottom lines of several cannabis businesses through reducing expenses, increasing production and improving product quality to the highest standards. Mr. Hoffman has also worked as a consultant in the cannabis industry for numerous years, focusing on work-flow, facility optimization, and new business development.
Prior to the cannabis space, Mr Hoffman spent more than 20 years in the Financial Technology & Services industry in roles ranging from CEO, Risk Manager, to Market Maker. Mr. Hoffman has also been involved as a co-founder in more than a half-dozen startups throughout his career. He holds a PhD in physics from Purdue University and studied at Cornell University and Northwestern University during his education.
"I am very pleased to be joining the Body and Mind team as the business enters a phase of significant expansion," stated Mr. Hoffman. "Along with the incredibly-talented team of experienced leaders, I am excited to bring my cannabis industry experience to further add value to the expansion of the Body and Mind operations and brand."
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this release.
About Body and Mind
BaM is a publicly traded company investing in high quality medical and recreational cannabis cultivation and production and retail. Our wholly-owned Nevada subsidiary was awarded one of the first medical marijuana cultivation licences and holds cultivation and production licenses in Nevada and partial ownership of a production and dispensary license in Ohio. BaM products include dried flower, edibles, topicals, extracts as well as GPEN Gio cartridges. BaM marijuana strains have won numerous awards including the Las Vegas Hempfest Cup 2016, High Times Top Ten, the NorCal Secret Cup and the Emerald Cup.
BaM continues to expand operations in Nevada and Ohio and is constantly reviewing accretive expansion opportunities.
Safe Harbor Statement
Except for the statements of historical fact contained herein, the information presented in this news release constitutes "forward-looking statements" as such term is used in applicable United States and Canadian laws. These statements relate to analyses and other information that are based on forecasts of future results, estimates of amounts not yet determinable and assumptions of management. Any other statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions or future events or performance (often, but not always, using words or phrases such as "expects" or "does not expect", "is expected", "anticipates" or "does not anticipate", "plans, "estimates" or "intends", or stating that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved) are not statements of historical fact and should be viewed as "forward-looking statements". Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such risks and other factors include, among others, the actual results of activities, variations in the underlying assumptions associated with the estimation of activities, the availability of capital to fund programs and the resulting dilution caused by the raising of capital through the sale of shares, accidents, labor disputes and other risks. Although the Company has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. There can be no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements contained in this news release and in any document referred to in this news release.
Certain matters discussed in this news release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved. Forward-looking information is subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company's ability to control or predict. Important factors that may cause actual results to differ materially and that could impact the Company and the statements contained in this news release can be found in the Company's filings with the Securities and Exchange Commission. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise. This press release shall not constitute an offer to sell or the solicitation of an offer to buy securities.
SOURCE Body and Mind Inc.
Premier Health Group to Expand Scope of Practice by Launching a Cannabis Clinic for Patients
VANCOUVER, Nov. 15, 2018 /CNW/ - Premier Health Group (CSE: PHGI) (OTCQB: PHGRF) (Frankfurt: 6PH) (the "Company" or "Premier Health"), a Company focused on developing innovative approaches that combine human skill based expertise with emerging technologies for the healthcare industry, is pleased to announce that as a part of its expansion plans, Premier Health will enter the Cannabis clinic space by a series of acquisitions, partnerships and/or launching a new Canada-based chain in the first half of 2019.
"The role of Cannabis in treating medical conditions is continuously expanding. Our doctors have had success treating their patients with various ailments from chronic pain to cancer related symptoms. Unfortunately, there is a gap between the patient's need for medical marijuana, and the family doctor's comfort and knowledge to prescribe it. We are looking to fill that gap with various forms of clinics and services to help our patients and healthcare workers," said Dr. Essam Hamza, Chief Executive Officer of Premier Health.
"Cannabis clinics fit in perfectly with our existing Telemedicine services and comprehensive app plans. The patient will not only be able to see their family doctor with our app, but also connect with their Cannabis clinic healthcare worker, the education nurse and eventually even the pharmacy or LP that is providing the prescription. This team based and encompassing approach will provide the best and most convenient care for our patients."
The Canadian medical cannabis clinic market is estimated to be worth approximately $2.35 billion by 2025.
The Company expects to provide additional updates on acquisitions in Q4-18 and Q1-19.
On Behalf of the Board of Directors
"Dr. Essam Hamza, MD"
Chief Executive Officer
About Premier Health
Premier Health is a Canadian company that is strategically poised to take advantage of business opportunities in the global health care industry. We are developing innovative health care approaches that combine human skill based expertise with emerging technologies, and will set the gold standard for services in locations of interest worldwide. Premier Health's subsidiary, HealthVue is focused on developing proprietary technology to deliver quality healthcare through the combination of connected primary care clinics with telemedicine and artificial intelligence (AI). We currently have an ecosystem of over 100,000 active patients and have plans to rapidly increase that number both domestically and internationally. The HealthVue team has a strong track record of successfully creating value in healthcare and technology enterprises. The Management team has deep clinical, financial and operational expertise and a passion for improving healthcare for all patients.
Cautionary Statements
This news release contains forward-looking statements that are based on Premier Health's expectations, estimates and projections regarding its business and the economic environment in which it operates, including with respect to the implementation of its shareholder communications initiative and the timing thereof. Although Premier Health believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and involve risks and uncertainties that are difficult to control or predict. Therefore, actual outcomes and results may differ materially from those expressed in these forward-looking statements and readers should not place undue reliance on such statements. These forward-looking statements speak only as of the date on which they are made, and Premier Health undertakes no obligation to update them publicly to reflect new information or the occurrence of future events or circumstances, unless otherwise required to do so by law.
The Canadian Securities Exchange does not accept responsibility for the adequacy or accuracy of this release.
SOURCE Premier Health Group Inc.





 PROVIDENCE, R.I. (November 15, 2018) – University Orthopedics announced that their team of sports medicine surgeons, Drs. Hulstyn, Fadale and Owens, have begun performing ACL repairs with BEAR, a procedure that has been developed by Dr. Martha Murray and her team at Boston Children’s Hospital, with the help of Dr. Braden Fleming and his team at Rhode Island Hospital.
PROVIDENCE, R.I. (November 15, 2018) – University Orthopedics announced that their team of sports medicine surgeons, Drs. Hulstyn, Fadale and Owens, have begun performing ACL repairs with BEAR, a procedure that has been developed by Dr. Martha Murray and her team at Boston Children’s Hospital, with the help of Dr. Braden Fleming and his team at Rhode Island Hospital.