Servier finalises acquisition of Shire's oncology branch
PARIS, Aug. 31, 2018 /CNW Telbec/ - Servier, an independent international pharmaceutical company, announced today that it has finalized the previously announced acquisition of Shire's oncology branch after obtaining regulatory clearance. The 2.4 billion dollar transaction will enable Servier to establish a direct commercial presence in the United States and strengthen its oncology drug portfolio in the countries in which the Group is already present.
Servier acquires Shire's oncology business including two marketed products, ONCASPAR® 1 and ONIVYDE® 2, in addition to two collaborations currently being developed in the immuno-oncology field.
"This acquisition is a major step in achieving the Servier group's ambition", declared Olivier Laureau, President of Servier. "It marks the launch of Servier's commercial activities in the world's largest pharmaceutical market - the United States - and significantly strengthens its portfolio of oncology drugs. It is part of Servier's twofold strategy: to continue to treat an increasing number of patients with innovative medications across the world and become a world reference in oncology."
In the United States, Servier products will be marketed by the Group's new subsidiary, Servier Pharmaceuticals LLC (Boston), which is made up of 80 people, including those employees transitioning from Shire to Servier. Servier Pharmaceuticals will be led by David K. Lee who previously led Shire's Global Genetic Diseases and Oncology franchises. The subsidiary will continue to market ONCASPAR® in the United States. Servier Pharmaceuticals' twofold aim is to develop a strong sales presence in the United States, based on Servier's existing portfolio, and to enrich the portfolio with close-to-market or marketed innovative products, in accordance with the Group's strategy.
Outside the United States, approximately 75 employees from Shire are expected to integrate into Servier's organizational structure. They will continue to market ONCASPAR®and ONIVYDE® according to local legislation.
With this acquisition, Servier takes another step toward its strategic ambition to become a global biopharmaceutical company.
About Servier
Servier is an international pharmaceutical company governed by a non-profit foundation, with its headquarters in France (Suresnes). With a strong international presence in 149 countries and a turnover of 4.152 billion euros in 2017, Servier employs 21,700 people worldwide. Entirely independent, the Group reinvests 25% of its turnover (excluding generic drugs) in research and development and uses all its profits for development. Corporate growth is driven by Servier's constant search for innovation in five areas of excellence: cardiovascular, immune-inflammatory and neuropsychiatric diseases, cancer and diabetes, as well as by its activities in high-quality generic drugs. Servier also offers eHealth solutions beyond drug development.
More information: www.servier.com
Follow us on Social Media:
https://www.linkedin.com/company/servier/
https://www.facebook.com/Servier/
https://twitter.com/servier
________________________
At Nearly 90, This Inspiring Man Shares Wisdom And Life Experiences We Can All Learn From
Pelham, NH, August 30, 2018 ― The story of Dr. Titus Plomaritis reads like a pitch for a Hollywood movie. The third of seven children born to Greek immigrants in Lowell, Mass., during the Great Depression, Plomaritis had to work on a chicken farm at age 12 to help support his family. From there, through pure gut determination and nose to the grindstone focus, he went on to make his mark on the football fields of his high school and college alma maters; serve his country as a US Army paratrooper; excel in the world of business; and become a highly respected doctor whose insight was sought out by President Jimmy Carter. From start to finish, this is a remarkable story of achieving the American dream. This is the story of Titus. And every word of it is true.
Titus is a nostalgia-filled autobiography packed cover to cover with remarkable memories and 250 photos — many from the White House — that support the motivational stories throughout the memoir. Plomaritis and Sam Weisberg, who was associated with the Lowell Sun for 60 years as a writer, librarian and Sunday sports editor, collaborated to write Titus. Winner of the Pete Delohery Award for Best Sports Book in the 2018 Shelf Unbound Indie Book Competition, Titus is an inspirational tale for young and old and everyone in between.
Author Titus Plomaritis was born in 1929 and became a living legend in Lowell, Mass., after leading the Lowell High School football team to its first undefeated season. Plomaritis would later play four seasons at Boston University. He served as a US Army paratrooper during the early post World War II occupation of Japan. He earned a doctor of chiropractic degree from the Chiropractic Institute of New York in 1957 and a master of science in nutrition from the University of Bridgeport in Bridgeport, Conn., in 1979. Plomaritis was a fulltime practicing chiropractor for over forty years.
He established the Plomaritis Family Foundation in 2012, which has funded over $70,000 in college scholarships for students graduating from Lowell and Pelham high schools. In addition, Plomaritis recently established a perpetual scholarship at Boston University. Plomaritis and his high school sweetheart, Claire, were married for sixty-five remarkable years and had four children.
Absolutely every dollar from the sale of Titus goes directly to the Plomaritis Family Foundation to scholarships for deserving students.
For more information, please visit http://titusplomaritis.com/.
Titus
Publisher: AuthorHouse
ISBN-10: 1477271392
ISBN-13: 978-1477271391
Available from Amazon.com, Barnesandnoble.com, Authorhouse, Boston University Book Store, Lowell High School Book Store, and National University of Health Sciences Book Store
| OLEO Appoints New Leadership Welcoming Skyler Bissell, Former Amazon and Boeing Employee, to the Role of Chief Executive Officer
Rapid company growth and demand at OLEO, Inc. calls for focused expansion and distinguished leadership naming former COO, Skyler Bissell, to the role of Chief Executive Officer for his entrepreneurial vision for branding, marketing of consumer goods, and cannabis industry insight.
SEATTLE, W.A. AUGUST 30, 2018 - OLEO, Inc. the industry leader in micro-encapsulation technology for the production of water-soluble powdered cannabinoids, announced this week that Skyler Bissell, former COO and co-founder at OLEO, will step up to the position of Chief Executive Officer, effective as of Monday, August 20th after a unanimous vote by OLEO’s board of directors. Under Bissell’s leadership, OLEO will continue pursuing technological excellence and innovation in their lines of powdered CBD beverages designed for health-conscious active lifestyles. [To Read the Full Press Release Hosted by PRweb Click Here] |
|
|
|
| Oh, What is OLEO? OLEO is an on-the-go powdered beverage mix infused with CBD! OLEO offers a Coconut Water Mix, antioxidant-packed Rooibos Tea Mixes, and a line of flavorless CBD powder called Original Mix, all designed to be water-soluble making them more bioavailable, potent and faster acting than the majority of CBD products on the market today. OLEO’s active ingredient - OleoCBD™ - is created with a patent-pending micro-encapsulation technology masking any bitter aftertaste and is optimized for your active lifestyle needs, showcasing its effects within 20 minutes.
Link: Further product info & media talking point highlights
Link: OLEO Hi Res Product Shots - Coconut Water Mix, Rooibos Tea Mix & OLEO Original Mix
Link: Image graphics explaining the scientific process, technology & The OLEO Advantage
Meet Every Runner’s New Beverage Best Friend, OLEO, the Powdered CBD Beverage Needed by Those Living an Active Lifestyle for
Optimal Physical Performance and Muscle Recovery
Taste the OleoCBD™ experience of Hydration without the ‘High’ - Infused with the most effective water-soluble CBD for endurance athletes, packed with naturally derived vitamins, minerals & electrolytes, without the counter-effective junk found in your average sports drink.
About the Company
OLEO, Inc. is a biotechnology company thoughtfully designing fast-acting, water-soluble CBD products with purpose and intention for the active lifestyle community. OLEO offers a collection of powdered beverages infused with their active ingredient OleoCBD™, including an all-natural Coconut Water Mix, an antioxidant Rooibos Tea Mix, and a flavorless CBD powder called Original Mix. OleoCBD™ is created with a patent-pending micro-encapsulation technology that masks any bitter aftertaste and showcases its effects within 20 minutes. With a provisional patent for the powdered cannabinoid formulation method filed in 2014, OLEO, Inc. was founded quickly thereafter in 2015 and began mass-producing micro-encapsulated cannabinoids for the hemp & cannabis markets by 2017. OLEO plays a tremendous role in the advancement of cannabinoid technology, testing standards, and consumer product offerings, helping to make the cannabinoid and plant medicine industry more trusted, beneficial and approachable for all. Further clinical study results regarding micro-encapsulation to be released in 2018 as per the OLEO team’s research influence and efforts. |
|
|
|
Ready-To-Go Organic Fruit Smoothies Unveiled at CHFA East 2018
Ceres Organic Juices introduces a new line of on-the-go fruit smoothies with real chunks of fruit and nothing but fruit
SAN DIEGO, California – (August 29, 2018): Ceres invites you to experience the fresh taste of 100% Organic Juices and On-the-Go Fruit Smoothies at booth #503 at the Canadian Health Food Association (CHFA) East, September 15-16, 2018. Located at Metro Toronto Convention Centre, South Building, Toronto, ON, CHFA East is Canada’s largest conference and trade show for the natural health and organics industry.
A brand long trusted to provide “Nature’s Perfect Juice”, Ceres introduces a mouthwatering new line of pure, non-GMO smoothies, Ceres Organic Smoothie to Go, featuring nothing but the best organic nutritious fruit. Each pouch delivers perfectly ripe, organic fruit into a healthy, sippable snack that is filled with the essential vitamins, minerals and nutrients your body craves without any fillers or added sugars.
Packed at the peak of freshness to preserve the pure, crisp taste of exotic real fruit from around the world, Smoothies to Go are the ideal way to get your “five-a-day” recommended servings of fruits and 100% of your daily Vitamin C. Each smoothie contains small chunks of luscious real fruit and comes in Mango, Tropical and Apple Berry flavors.
Experience the pure taste of Ceres Organic 100% Fruit Juice and Smoothie to Go at CHFA East 2018, booth #503. Visit www.organicceresjuices.com to learn more about Ceres Organic’s range of products.
About Ceres:
At Ceres Fruit Juices, we believe that when we are good to the earth, she returns her goodness in the rich, full flavors of her fruit, and we are delighted to bring this natural bounty to our customers just as Mother Nature intended - in our line of organic, non-GMO, gluten-free, vegan and kosher juices and smoothies that are free of added sugar or preservatives. From humble beginnings in South Africa’s Ceres Valley, Ceres Fruit Juices now provides all-natural, organic juices internationally. Fans in the U.S. and abroad choose Ceres Juices for their healthy ingredients that taste freshly picked. Each bottle or pouch is made from a careful selection of the finest quality organic fruits that meet only the highest farming standards. Learn more about our company and our premium fruit products at www.CeresJuices.com.
Rock Solid Research On How To Prevent Dementia And Maintain A Healthy Brain
Chattanooga, TN, August 30, 2018 ― Dr. Timothy R. Jennings speaks expertly on a subject that concerns over 5.5 million people across the nation: how to prevent dementia and keep our mind sharp as we age. A psychiatrist and international speaker, Jennings introduces his new book, recently rated #1 by Amazon in books on dementia, The Aging Brain: Proven Steps to Prevent Dementia and Sharpen Your Mind.
Dr. Jennings prescribes simple, everyday actions we can take to stave off disease, promote vitality, and prevent dementia and late-onset Alzheimer's. "The choices we make now can help us to keep our minds sharp and maintain our independence as we age,” says Jennings.
An easy-to-use guide to maintaining brain and body health throughout life, The Aging Brain is based on solid, up-to-date scientific research, and the interventions discussed can prevent progression toward dementia, even in those already showing signs of mild cognitive impairment. The recommendations also may help reduce disability and depression.
"This book isn't just for people hoping to slow the aging process,” says Jennings. "It's also for anyone who is a caregiver to someone at risk of or already beginning to suffer from dementia. It offers a hopeful, healthy way forward.”
Jennings, who maintains a private practice in Chattanooga, TN, has authored several books, including The God-Shaped Brain and The God-Shaped Heart. He is a Distinguished Fellow of the American Psychiatric Association and Fellow of the Southern Psychiatric Association, and is president and founder of Come and Reason Ministries.
For more information about Dr. Jennings, please visit the website: https://www.agingbrainbook.com.
To connect with Dr. Jennings, please visit: https://www.facebook.com/DrTimJennings/ and https://twitter.com/timjenningsmd.
The Aging Brain: Proven Steps to Prevent Dementia and Sharpen Your Mind
Baker Books
Released: June 2018
ISBN-10: 080107522X
ISBN-13: 978-0801075223
September is Ovarian Cancer Awareness Month
Common Symptoms Could Lead to Serious Diagnosis:
What Women Need to Know About Latest in Ovarian Cancer Treatment
Back pain, bloating, and fatigue…what woman hasn’t experienced these symptoms at least once in her lifetime?
As women age, some may write off aches and pains as daily routine. Unfortunately, when these common symptoms persist, they could be a serious sign of ovarian cancer.[i] Women may also be surprised to know that even though the rate of ovarian cancer has been slowly falling over the past 20 years[ii]:
- There are approximately 225,000 women in the US living with ovarian cancer, many of whom were diagnosed when their disease was already in advanced stages
- About 14,070 women will die from ovarian cancer this year
- About 22,240 will receive a new diagnosis of ovarian cancer
- Only 20% of ovarian cancers are found at an early stage[iii]
- Approximately 85% of women with advanced ovarian cancer will recur after treatment[iv], and once recurrence occurs, the disease is considered incurable
The good news is that new treatments, known as maintenance treatments, are now available for women whose disease has recurred and has either fully or partially responded to chemotherapy. These treatments can help extend the time between potential recurrences, also known as progression-free survival. While listening to your body and knowing the symptoms of ovarian cancer is crucial for early diagnosis, these new treatments help to empower women by providing new options to delay disease progression when previously, observation was the only option.
On Wednesday, September 12 in time for Ovarian Cancer Awareness Month leading gynecologic oncologist Dr. Robert Holloway will discuss more about new treatments for recurrent ovarian cancer. Joining will be Anne, who will share her personal experience with the disease, and how she was able to persevere and overcome challenges despite her diagnosis.
Plastic Surgeon Gets Real on the Secrets Most Keep Under Wraps
With poplar TV shows such as “Botched” and the old favorite NipTuck, plastic surgeons are seen as the perfect specimen of looks, wealth and a fabulously crafted and curated lifestyle. They’re impeccably dressed, have gregarious personalities and their significant others are the epitome of perfection. Is there more to the story? Are there practice secrets that are kept more confidential than the facelift of a 50-year-old socialite? Dr. Stanley Poulos, a San Francisco Bay area board certified plastic surgeon gets real on secrets many plastic surgeons prefer to keep under wraps.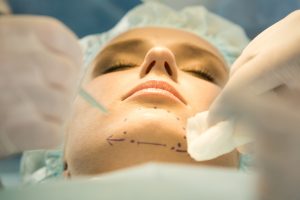
1)Sometimes you will have more than just “a little discomfort” managed with some light dosage of pain medication.
Everyone has a different pain threshold and post op bruising and swelling vary so certain procedures will take longer to recover from. Abdominoplasty, body lifts, and combination surgeries like “mommy makeover” are just a few examples of procedures where recovery may be extended.
Many people who have had procedures can think of a time a few days post op where they wanted to curse their surgeon or ask “why in the world did I do this?”
Just because it’s an elective aesthetic surgery doesn’t make it any less of an operation. Dr. Poulos explains that there are two very important things you want to get clear on, the actual level of pain and recovery time. Recovery time to return to ranching or running marathons may be a lot different than being able to drive or get out. He advises speaking to a few friends or references who had the same procedure. Just remember that like childbirth as time goes by the experience may seem easier than it was.
“You need to know so you can make necessary arrangements with work and childcare or elder care if need be. Some procedures can take at least a week or two before resuming normal activity so the more realistic the expectations the better,” adds Poulos.
2) There may be more than just “minimal scarring.”
“Scarring has nothing to do with the surgeon’s skill and everything to do with the patient’s genetics,” clarifies Dr. Poulos. He goes on to explain that a highly skilled plastic surgeon can do the same exact procedure with closure on two different people. One will have minimal scarring while the other will not.” People with darker complexions should consider this before deciding to do a procedure and certainly consult with your doctor’s team who can recommend remedies that help with scarring.
3) Cellulite removal isn’t just easily “zapped away” with a laser.
Today there are many options available, each claiming to treat cellulite effectively. Some are a bit more invasive. Cellulaze for example is a highly promoted actual surgical procedure using an invasive laser that must be threaded under the skin. “Even though this is considered minimally invasive, it’s not an easy zap. Even with improvement the results often will not meet expectations so be realistic.
4) There’s an expiration date on results due to the aging process.
We see celebrities who desperately battle mother nature. Aging is inevitable. The average "shelf life" of a facelift is about 10 years. “A facelift is a large financial investment along with significant recovery time. The better the skin elasticity the closer to a decade you’ll get. Odds are even better you’ll have a more enduring result if you don’t smoke or sun-worship,” says Dr. Poulos. He goes on to say that he believes it’s really important to lay out the real expectations given skin texture and volume. Some people may notice the lower quadrant of their faces aging just a few years post facelift. While a person will still look youthful post facelift, it’s not going to prevent aging. As they say “ageing is not for sissies but it sure beats the alternative.”
About the Doctor:
Dr. Stanley Poulos is a Board-Certified Plastic Surgeon and Co-Founder with Yngvar Hvistendahl, M.D of Plastic Surgery Specialists recognized as one of the premier aesthetic surgery clinics in Northern California, with over 30-years’ experience in aesthetic procedures, and mastery of facial and body symmetry. Dr. Poulos offers weight loss procedures such as the gastric balloon that allow patients to achieve optimal health and, when combined with exercise and proper nutrition, and ongoing consultation with a specialist (an option few surgeons offer before considering surgery) the results have been exceptional.
A graduate of the University of Texas Medical School, Dr. Poulos completed his internship and residency at UC San Francisco. He completed surgery and plastic surgery training in San Francisco prior to entering private practice in Marin County where he co-founded PSS (www.psspecialists.com)
BlueWind Medical Announces Positive Long-Term Results of the BLUEWIND-RENOVA™ Implantable Tibial Nerve Neuromodulator
Results from 36-month OPTIMIST Follow-Up study show BlueWind Renova™ is a safe, minimally invasive system that offers durable and significant improvements for Over Active Bladder patients
HERZLIYA, Israel, Aug. 30, 2018 /CNW/ - BlueWind Medical, developer of the RENOVA iStim™, an innovative, leadless, miniature, wireless neurostimulation platform, for the treatment of multiple clinical indications, announced today the publication of long-term clinical results of OPTIMIST follow-up study. BlueWind Medical is the only company today with long-term positive clinical data for implantable tibial stimulation for the treatment of overactive bladder (OAB). An estimated 66 million people in the EU and 43 million in the US suffer from OAB, a disease that adversely affects patients' quality of life.
The prospective study was conducted in four leading clinical centers in the UK and the Netherlands that evaluated the long-term performance and safety of the RENOVA iStim™ for the management of OverActive Bladder (OAB), including Urinary Urge Incontinence (UUI) and symptoms of Urgency Frequency (UF).
The OPTIMIST Follow-Up study is an extension of the previously published OPTIMIST six-month pilot study and included 20 of the 36 pilot study patients. The 20 patients that were available for long-term follow-up well represent the original pilot cohort based both on demographics and on clinical response.
The study demonstrated a sustained high responder rate over a 36-month follow-up period, comparable with response rates typical to sacral neuromodulation. Three years after implantation of the RENOVA iStim™ device, 75% of patients experienced at least a 50% long-term reduction in OAB symptoms. Patients experienced durable, long-term, effect of UUI relief in "leaks" (50% of patients) and in "large leaks" (80% of patients). No severe adverse events (SAE's) were reported throughout the follow-up study.
"We are very encouraged by the sustained long-term results of the RENOVA system", said Dr. John Heesakkers, the primary investigator of the study. "The study provides assurance that BlueWind's RENOVA iStim stimulation of the tibial nerve can lead to meaningful symptoms improvement, without the clinical risks associated with sacral device implantation".
The study also demonstrated that the RENOVA iStim™ system provided long-term improvement in all health-related quality of life aspects of patients, with 70% of the patients showing a meaningful improvement as assessed by OAB quality of life questionnaire.
"The 36-month study results confirm that the RENOVA iStim™ system provides robust and effective long-term performance, that could help millions of patients in the management of OAB", commented Efi Cohen Arazi, CEO of Rainbow Medical and Chairman of the Board of BlueWind Medical. "We believe BlueWind has highly differentiated wireless neuromodulation, and this data provides strong evidence of the potential for this technology in OAB and other indications".
About BlueWind Medical
BlueWind Medical (http://www.bluewindmedical.com ) was founded in 2010 by Rainbow Medical. The company is developing a platform technology of miniature, wireless, neurostimulators that can be injected or implanted in a minimally invasive procedure to treat multiple indications. By putting patients' needs first, BlueWind Medical's team of experienced and dedicated engineers and researchers are creating a versatile and effective platform that will transform neuromodulation as we know it.
About Rainbow Medical
Rainbow Medical (http://www.rainbowmd.com ) is a unique private operational investment company that seeds and grows start-up companies developing breakthrough medical devices, addressing significant unmet market needs in a diverse range of medical fields.
SOURCE BlueWind Medical
Crop Subsidiary to Open Italian Retail Locations Launching its First Line of Hemp Oil Infused Products in Italy
VANCOUVER, Aug. 30, 2018 /CNW/ - CROP Infrastructure Corp. (CSE: CROP) (OTCMKTS: CRXPF) ("CROP" or the "Company") announces it will launch its first line of Hemp oil infused cosmetic and therapeutic products under the brand 'URBAN JUVE', pursuant to its previously announced License Agreement with The Yield Growth Corp.'s subsidiary, Urban Juve Provisions Inc. (formerly Juve Wellness Inc.). The License Agreement gives CROP exclusive rights in Italy to the URBAN JUVE products, as well as non-exclusive distribution rights in the United States.
Furthermore, the Company in partnership with the team from Xhemplar S.R.L. CROP's cultivation and extraction joint venture partner in Italy, is scouting locations to open 2 CBD retail outlets in Northern Italy under the company's Emerald Heights brand, before the end of 2018.
The URBAN JUVE product line which will be featured prominently along with Xhemplar products, and Hempire hemp oil products at all Italy locations. Urban Juve is inspired by Ayurvedic philosophy and is created for the modern, wellness-conscious consumer. The unique formulations benefit consumers seeking natural products made with the highest quality ingredients. Urban Juve is manufacturing its first line of 12 topical products in the fall of 2018. All the products contain hemp oil procured through a patent pending hemp oil extraction process. Crop has the right to add hemp oil to the products and distribute them throughout Italy.
According to a study by Arcview Market Research and its research partner BDS Analytics, by 2027 worldwide sales of legal cannabis are forecast to reach $57 billion. During that period, spending in North America is expected to leap from $9.2 billion to $47.3 billion driven mainly be recreational use. The fastest cannabis market growth is expected to come from outside North America, especially Europe where the main growth driver will be medical applications. Medical cannabis use will be fed by $1.3 trillion estimated annual government-subsidized healthcare spending. The structure of the healthcare industry is expected to make Europe the number one medical cannabis market in the world.
"We are excited to be partnered with CROP for the European launch of the URBAN JUVE hemp oil infused product line," says Penny Green, President and CEO of Yield Growth. "CROP is a demonstrated leader in the international hemp market with affiliations to hemp production in Nevada, California, Washington, Italy and Jamaica."
CROP Infrastructure Director & CEO Michael Yorke, states: "We are pleased with the URBAN JUVE branding initiative by Yield Growth and feel it will resonate with health and lifestyle consumers. We look forward to offering the URBAN JUVE products in Italy."
About CROP Infrastructure Corp.
CROP Infrastructure Corp. is publicly listed on the Canadian Securities Exchange and trades under the symbol "CROP" and in the U.S. under the symbol "CRXPF". CROP is primarily engaged in the business of investing, constructing, owning and leasing greenhouse projects as part of the provision of turnkey real estate solutions for lease-to-licensed cannabis producers and processors offering best-in-class operations. The Company's portfolio of assets includes cultivation properties in California, Washington State, Nevada, Italy, Jamaica and a joint venture on West Hollywood and San Bernardino dispensary applications. CROP has developed a portfolio of 16 Cannabis brands and has U.S. and Italian distribution rights to a line of over 55 topical cannabis products from The Yield Growth Corp.
Disclaimer for Forward-Looking Information
Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events, and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. In addition, marijuana remains a Schedule I drug under the United States Controlled Substances Act of 1970. Although Congress has prohibited the US Justice Department from spending federal funds to interfere with the implementation of state medical marijuana laws, this prohibition must be renewed each year to remain in effect. These statements generally can be identified by the use of forward-looking words such as "may", "should", "could", "intend", "estimate", "plan", "anticipate", "expect", "believe" or "continue", or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding the expected yield from The Jamaica Property; the technological effects of The Jamaica Property on production; the intention to expand its portfolio; and execute on its business plan. Such statements are qualified in their entirety by the inherent risks and uncertainties surrounding the regulatory and legal framework regarding the cannabis industry in general among all levels of government and zoning; risks associated with applicable securities laws and stock exchange rules relating to the cannabis industry; risks associated with maintaining its interests in its various assets; the ability of the Company to finance operations and execute its business plan and other factors beyond the control of the Company. Such forward-looking statements should therefore be construed in light of such factors, and the Company is not under any obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
SOURCE CROP Infrastructure Corp.
Learn to love the burn
Trick your brain into enjoying better workouts
Getting into shape isn’t just about toning and strengthening the muscles in your body. Turns out, your brain is the most important body part when it comes to exercise. As the command centre, your brain sends messages to muscles to tell them to move, it also moderates mood, energy levels, and motivation.
During an intense workout, the brain is programmed to read the experience as pain or discomfort, which can translate into negative, irrational thoughts about your workout. But according to Scott Leith, PhD, data scientist and research psychologist with GoodLife Fitness, you can trick your brain to push past the negative and switch over to enjoying exercise – which translates to longer, more intense workouts and helps make exercise a habit.

Leith suggests some easy ways to reframe exercise for better results:
- Distraction. It seems simple, but distracting yourself with TVs, upbeat music or an audiobook can distract your brain's focus and allow you to push harder or longer.
- Positive imagery. It can be motivating to visualize yourself once you’ve reached your fitness goals – healthier, stronger and maybe returning to sports or fitting into clothes you could never wear before.
- Motivational self-talk. Research shows positive self-talk can improve endurance exercise performance and lower perceived exertion. Leith suggests adjusting your positive self-talk to fit the stage of exercise (ramp up/psych up, intense phase support, ramp down).
- Identify and minimize negative or self-defeating thoughts or self-talk. When our minds feel pain or discomfort we tend to overgeneralize and amplify negative thoughts. Instead try noticing when you feel bad, identify the negative thought, then reframe them into positives.
- Channel frustration into effort. Instead of giving in to your frustration and negative thoughts, try pushing yourself harder. If you do it repeatedly, your brain will switch gears automatically and just keep going.
- Set small goals. Don’t try to do push yourself too far when you first start a new work out. Set smaller goals then work your way up. This means “Next week, I’ll add 5 lbs to my barbell,” or “I’ll just focus on making it two more minutes.” Increase your targets each time and your brain will adjust to the effort and send fewer negative signals.
Scott Leith is available to speak more about how to trick your brain into enjoying physical activity more.
Also, certified personal trainers in your region can demonstrate how to use mind tricks to push through some of the most common exercises we dread – burpees, cardio, box jumps, push-ups and more.


