Media Advisory - Canadian Centre on Substance Use and Addiction Presents a National Conference on Prevention, Harm Reduction, Treatment and Recovery
CALGARY, Nov. 9, 2017 /CNW/ - Issues of Substance (IOS) is Canada's only national conference that brings together addiction workers, healthcare professionals, researchers, policy makers, knowledge brokers and those with lived and living experience from across the country.
This three-day event is hosted by the Canadian Centre on Substance Use and Addiction (CCSA). The presentations, workshops and keynote panel discussions scheduled for this conference were carefully chosen to reflect the latest research, innovative ideas and urgent issues affecting Canadians. The conference theme is Addiction Matters.
Conference Details:
| What: |
Issues of Substance Conference |
| When: |
November 13–15, 2017 |
| Where: |
Hyatt Regency Calgary, 700 Centre Street S., Calgary, Alberta |
| Website: |
www.issuesofsubstance.ca |
We are pleased to announce that the Honourable Brandy Payne, Alberta Associate Minister of Health, will be speaking during the opening remarks on Monday. On Wednesday, the Honourable Ginette Petitpas Taylor, federal Minister of Health, will be delivering a closing address. For full details, please see the schedule below.
Media are invited to attend; please see the accompanying schedule. Onsite, accredited media are to identify themselves at the registration desk to receive their media pass, which must be visible at all times. Media are encouraged to RSVP in advance to media@ccsa.ca.
Conference Events Open to Media
| Date |
Session Title and Speakers |
Time (MST) |
| Mon., Nov. 13 |
Opening Remarks and Welcome
Rita Notarandrea, CEO, Canadian Centre on Substance Use and Addiction (CCSA)
Michael Prospero, Board Member, Canadian Centre on Substance Use and Addiction
Honourable Associate Minister of Health, Brandy Payne, Government of Alberta |
8:30—
9:00 a.m. |
| Mon., Nov. 13 |
Keynote Address: Alberta Family Wellness Initiative: Can One Story Change Everything to Improve Health and Well-being Outcomes?
Presented by Nancy Mannix, Palix Foundation |
9:00—
10:00 a.m. |
| Mon., Nov. 13 |
iPoliticsLIVE Panel: Good Health as We Age: Perspectives on Substance Use and Aging
Panelists:
Rita Notarandrea, CEO, CCSA
Franco Vaccarino, CCSA's Scientific Advisory Council, University of Guelph
Alastair Flint, University of Toronto
Tony George, CCSA's Scientific Advisory Council, Centre for Addiction and Mental Health, University of Toronto |
1:15—
2:45 p.m. |
| Tues., Nov. 14 |
Keynote Panel: Coming to Grips with the Opioid Crisis
Moderator: Jane Buxton, BC Centre for Disease Control
Panelists:
Dr. Norman Buckley, McMaster University
Dr. Nicholas Etches, Alberta Health Services
Dr. David Juurlink, Institute for Clinical Evaluative Sciences, University of Toronto
Donna May, mumsDU |
9:00—
10:00 a.m. |
| Tues., Nov. 14 |
Public Release of the National Alcohol Strategy Monitoring Project: A Status Report with Dr. Amy Porath, CCSA |
10:30—
10:35 a.m. |
| Wed., Nov. 15 |
Addressing the Opioid Crisis: Lessons Learned from the United States — Presented with support from the United States Embassy
Moderator: Peter Selby, First Do No Harm Co-chair, Centre for Addiction and Mental Health
Panelists:
Dr. Jane Maxwell, University of Texas at Austin
Dr. Robin Pollini, Department of Behavioral Medicine and Psychiatry, West Virginia University School of Medicine
Kelly J. Clark, American Society of Addiction Medicine |
8:30—
10:00 a.m. |
| Wed., Nov. 15 |
Keynote Panel: Happy Hour: Promoting a Culture of Moderation
Moderator: Ann Dowsett Johnston, Author
Panelists:
Tim Stockwell, Centre for Addictions Research of BC
Hubert Sacy, Éduc'alcool
Beth Martin, Nova Scotia Liquor Corporation |
10:30—
11:45 a.m. |
| Wed., Nov. 15 |
Closing Remarks
Includes public release of the Joint Statement of Action: A Year in Review report
Honourable Petitpas Taylor, Minister of Health
Rita Notarandrea, CEO, CCSA |
11:45 a.m.—
12:15 p.m. |
SOURCE Canadian Centre on Substance Use and Addiction
Adult Acne: Which Product Option is Right for You?
There are so many acne and breakout busting treatments on the market. It’s common to get a feeling of product overload. Dr. Magarita Lolis Dr. Margarita Lolis a Board-Certified Dermatologist in northern New Jersey who takes a holistic approach to treating skincare issues; breaks down the list of common products to consider using with the benefits of each. Hopefully this menu of options will clear some confusion.
Go simple on cleanser. People who are prone to breakouts may use a cleanser with salicylic acid triggering excessive dryness which only increases oil production leading to a breakout cycle. Gentler products that are appealing to all skin types such as Cetaphil or CeraVe are great options because they don’t strip away moisture and soothe the face.
Exfoliate weekly. They key here is not to be too harsh. You want to gently rub product into your face in circular motion then rinse with luke warm water. There are new products available with charcoal an ingredient which does a great job controlling oil in acne prone skin. People with sensitive skin should opt for an exfoliating mask which is less irritating.
Soothe with serums. Acne prone skin needs moisture not oil. Serums that address acne without compromising moisture are a great bet. Serums penetrate the skin quickly making them a great option for nighttime healing as you sleep.
Try skin oils. You can add drops of vitamin C oil, argan oil, or vitamin e oil to your serum or directly onto your face after cleansing which is like a vitamin boost to skin. This will address acne scarring helping them heal quicker.
Don’t skip moisturizer. Many people who are prone to breakouts want to skip moisturizer because they think their skin is oily enough. The reason skin is producing oil is because it is lacking moisture. Moisturizers with Hyaluronic acid draws water from the atmosphere and keeps skin hydrated. This is great for all skin types. You cannot go wrong with more water in the skin.
Consider a peel with a pro. Chemical peels are a great option for skin that has scarring and
Hyperpigmentation. While there are at home, do it yourself options available I have seen many people harm their own skin with peels. You really want to work with a professional as not to make matters worse. People often think a burning sensation means it’s working so they mistakenly leave peels on for too long and hurt themselves.
Balance skin with a toner. I like toners because they do a nice job of clearing away dirt and make-up, shrinking pores, and restoring skin to its natural pH balance. Witch Hazel is a simple no fuss astringent found at any drug store which works wonders on acne or break out prone skin.
Do a mask every week. You want to choose “detoxifying masks” featuring ingredients like, charcoal, clay, sulfur. You may want to alternate between a more soothing mask one week and a treatment mask when a breakout first occurs. Another option is using a soothing mask on the cheeks and then a clarifying mask on the chin and jawline.
Spot treat and refrain from picking. We touch our faces approximately 25 times per hour. It’s hard to be conscious of it. When we have a breakout, we may sit at our desks or behind the wheel picking away. It is a habit that spreads bacteria and leads to scarring. Getting a prescription level topical spot treatment is key because many are designed to work immediately which lessens the need to pick.
Ultimately, when in doubt see your dermatologist who will assess your skin and the many factors that may be causing acne. Oftentimes dermatologists will outline a treatment regimen that is more customized to you considering age, skin type and lifestyle.
Dr. Margarita Lolis, M.D. is a board-certified cosmetic, medical dermatologist and a fellowship-trained Mohs surgeon with over 20 years of experience. In her practice, she addresses common skin concerns such as acne prevention and treatment in both teens and adults, sun-damage, skin discoloration, wrinkles, changes to skin texture and loss of volume. On the medical side, she is a trusted expert in melanoma and over-all skin health. Dr. Lolis prides herself in honoring facial symmetry to deliver a natural look to her clients. She always recommends a healthy skin care regimen plus lifestyle habits that are aligned with her holistic approach to beauty. Dr. Lolis is a member of the American Academy of Dermatology, American College of Mohs Surgery, and the American Society of Anti-aging. Her practice, Skin, Laser, and Surgery Specialists is in New York City and Bergen Country, New Jersey.
SPINCO SUPPORTS MOVEMBER
Throughout the month of November, SPINCO will be supporting Movember with the below initiatives, and encourages the local community to join them and get involved!
SPINCO TORONTO MOVEMBER - TEAM PAGE
SPINCO has created a team page at movember.com, where they are inviting you to join them in raising money for this incredible cause. All of SPINCO's male instructors will be participating by growing moustaches, too!
SPIN-IT-FORWARD
For the month of November, all proceeds from SPINCO's Spin-It-Forward classes every Monday at 6:30pm will be donated to Movember.
SPINCO MOVEMBER MOUSTACHE METER
SPINCO has set a fundraising goal of $5,000, which will be tracked throughout the month on their SPINCO Movember Moustache Meter at the studio!
END OF MONTH CELEBRATION
At the end of the month, SPINCO will host a celebratory ride followed by snacks, drinks and music in-studio. A barber will also be present for anyone that wants to ditch their 'stache!
Will marijuana play a role in the future of American politics?
If people let government decide which food they eat and medicines they take, their bodies will soon be in as sorry a state as are the souls of those who live under tyranny.
– Thomas Jefferson
“Ready or not, marijuana is fueling massive change and has become a mainstream topic of significant importance to people of all ages in most households across America,” says Dr. David Bearman, expert in the history and role of marijuana. “Right now, all but four states have legalized at least some form of medical cannabis and the legalization of recreational use is growing faster than ever before.
“While neither political party has made marijuana a major centerpiece for discussion, marijuana politics stands to make a major difference in state and national political outcomes in the next elections.”
Dr. Bearman says that medical marijuana matters to the young, middle aged, and the old.
“It is more widely used, enjoyed, and appreciated than any of its detractors is willing to admit. There are numerous political candidates seeking to reform federal marijuana policies - from pushing to protect state laws to advocating marijuana's reclassification from a Schedule I drug like heroin to Schedule III drug so long term studies can be done on the drug's medical benefits. Cannabis reform is now occurring on multiple fronts.”
An August 2016 study published by Harvard Medical School identifies over 20 million people use medical marijuana in the United States.
A partial list of the known medical marijuana uses now includes:
- As an appetite stimulant in AIDS and chemotherapy patients
- To relieve pain (and its use is being recommended to doctors in lieu of prescribing opiates)
- To help treat inflammatory bowel diseases like Crohn’s disease and ulcerative colitis
- To treat chemotherapy-related nausea and vomiting
- To treat muscle spasticity and pain in multiple sclerosis
- To reduce the growth of cancers
- To treat cancer-related pain not managed by other pain medication
- To treat drug-resistant epilepsy, particularly in children
- To treat psychiatric disorders (e.g., anxiety, depression, PTSD, substance use disorders, and bipolar disorder)
- To reduce the symptoms of conditions in the autism spectrum disorder
- To reduce the side effects of treatment for Hepatitis C (nausea, fatigue, muscle aches, and depression).
- To reduce the symptoms of autoimmune disease (e.g. Rheumatoid arthritis, ulcerative colitis, fibromyalgia, Restless Leg Syndrome, Reflex Sympathetic Dystrophy, Complex Regional Pain Syndrome)
- To help people get to sleep, get better quality sleep and awaken without a drug hangover
Although marijuana can help relieve the symptoms of many medical conditions and is used as medicine most states, its use is still prohibited by federal level. In something of a Catch-22, the DEA and FDA complain that there is no data from large, long-term, well-designed studies. In order to say that, they have to ignore at least 20 tincture of cannabis/Nabixamol studies that have been done in the UK.
These double-blind studies can only be legally done with the government’s cannabis, and the government rarely approves such studies. Why? Marijuana was made illegal in 1970s for political reasons and similar political reasons remain. The DEA seems more concerned with job protection than science or truth. The Federal Drug Administration continues to have concerns about potential risks versus benefits.
Forty-six states have passed laws, contrary to federal laws, to allow the use of marijuana for medical conditions. States have also made the move to decriminalize recreational marijuana use by adults or have similar measures on upcoming ballots.
“Medical and recreational marijuana is going to energize large portions of the voting populace, particularly among students, those looking for alternative health care options, and minorities,” Dr. Bearman says. “The growing awareness that marijuana is medicine is forcing change.”
One thing is for certain. Any attempts by Donald Trump and Attorney General Jeff Sessions to stamp out further legalization efforts is going to be met with a loud public outcry. Their efforts would also be unconstitutional because of the states rights guaranteed by the 9th and 10th amendments as well as the 1925 SCOTUS decision in Linder v. United States that confirmed the regulation of the practice of medicine is a right reserved for the states.

Drugs are Not the Devil’s Tools
Dr. David Bearman
Color $65.00 B&W $45.00
Revised and updated November 2017
8 ½ x 11, 498 pages
Entertaining, lavishly illustrated and full of amazing stories, Drugs are NOT the Devil’s Tools takes the reader through the history of drugs and the origin of the onerous United States drug laws.
Dr. David Bearman shows how, through intertwining motives of discrimination and greed, often under the guise of morality, how those in power have created a drug policy that is completely dysfunctional. He details how drug laws have been very effective in further marginalizing discriminated-against groups and have been a total failure in every other respect.
Additionally, did you know:
- We all have cannabis receptors in our bodies
- Cannabis was legal in the US until 1943 and only removed because the powers that be wanted to get rid of hemp and create a market for nylon and other petroleum-based products
- The American Medical Association opposed the removal of cannabis from pharmacies
- There is no such thing as cannabis addiction
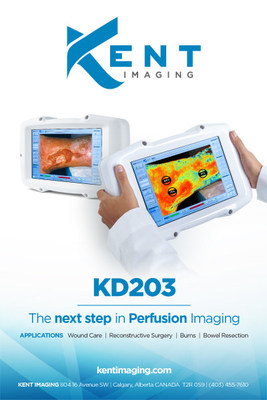
Kent Imaging's Breakthrough Medical Imaging Device Licensed with Health Canada
CALGARY, Nov. 7, 2017 /CNW/ - Kent Imaging Inc., a leading innovator in multispectral oxygenation imaging, announced today that their handheld KD203 is a licensed medical device with Health Canada. Kent's device is also cleared with the U.S. Food and Drug Administration (FDA) and the company is ISO13485 certified.
Kent's handheld KD203 measures and displays quantified tissue oxygen saturation levels in superficial tissue. Tissue oxygen saturation measurements provide critical information to help physicians determine at risk tissue and guide treatments to optimize patient outcomes. Kent's easy to use device is available for sale in the United States and Canada with utility in plastic surgery, colorectal surgery, trauma, wound care, limb salvage, podiatry, burns and cardiac specialties.
"The Kent Imaging camera is unique in its ability to assess oxygenation in tissue using spectroscopy," said Dr. Earl Campbell MD, FRCSC, practicing surgeon and a member of the Alberta Medical Association and the Canadian Society of Plastic Surgeons. "Plastic surgeons in Calgary, Alberta are already exploring its intra-operative uses when performing breast reconstruction procedures."
"We are happy to announce our licence with Health Canada," said Pierre Lemire, CEO of Kent Imaging. "This, along with its FDA clearance, has made our handheld device, the KD203, readily available to the physicians and surgeons who require the valuable insight it provides."
Features of Kent's KD203:
- Instant and precise visualization of oxygen saturation
- Non-invasive with no injectable dyes
- Ability to image the actual wound bed and surrounding tissue
- Oxygenation measurement of surgical sites or reconstructive flaps
- A means to track patient progress and treatment effectiveness
- Ease-of-use with minimal training required
- Accurate, unlimited imaging
- Lightweight and completely portable handheld device
About Kent Imaging Inc.
Kent Imaging Inc., a leading innovator in multispectral oxygenation imaging, designs, manufactures, and markets imaging technology to improve health care. The team at Kent Imaging is committed to improving patient outcomes by delivering innovative and practical solutions for the healthcare industry. Kent Imaging holds multiple patents in medical technology and is currently commercializing the breakthrough multispectral imaging solutions that assesses tissue oxygen saturation. The company is headquartered in Calgary, Alberta, Canada, and has locations in Chicago, Los Angeles, Salt Lake City and Seattle.
Kent's KD203 is U.S. Food and Drug Administration 510(k) cleared, and Health Canada licensed. The device is available for sale in the United States and Canada. Improving on Kent's previous KC103, the handheld Kent KD203 is intended for use by healthcare professionals as a non-invasive tissue oxygenation measurement system that reports oxygen saturation (StO2), relative oxyhemoglobin level (HbO2), and relative deoxyhemoglobin (Hb) level in superficial tissue. Kent's multispectral imaging technology displays two-dimensional color-coded images of tissue oxygenation of the scanned surface and reports multispectral tissue oxygenation measurements for selected tissue regions. Tissue oxygen saturation measurements provide critical information to help physicians determine at risk tissue and guide treatments to optimize patient outcomes.
SOURCE Kent Imaging
Think Research to deliver comprehensive healthcare solutions on the IBM Cloud
TORONTO, Nov. 6, 2017 /CNW/ - Think Research, a leading digital healthcare platform provider, today announced that it has partnered with IBM to drive further scalability for their suite of cloud-based clinical decision support tools using IBM Cloud.
To address modern medical challenges, health providers need access to the best clinical knowledge in easy-to-use and well-designed tools. Think Research provides this executable knowledge through a broad collection of health care applications, including Patient Order Sets, eForms, medication reconciliation, progress notes, eReferrals and more.
"We're dedicated to using modern tools to bring transformational change to the healthcare industry," said Saurabh Mukhi, Chief Technology Officer at Think Research. "Our suite of health care applications provide solutions across the health care spectrum. From primary providers and acute care settings through to long-term care facilities, we know their diverse needs demand the best infrastructure technology available."
To support their expanding global customer base, Think Research partnered with IBM to help deliver the robust technical infrastructure their highly-regulated customers' demand, while maintaining world-class security. IBM Cloud will help Think Research scale with success without compromising privacy concerns.
"Healthcare organizations need efficient and sustainable solutions to address this industry's challenges in new and innovative ways," said Nathalie Le Prohon, vice president of IBM's healthcare industry in Canada. "IBM Cloud provides a secure and reliable platform for healthcare companies, such as Think Research, to deliver better outcomes and drive much-needed transformation."
About Think Research
Think Research's mission is to organize the world's health knowledge so everyone gets the best care. The company takes the ever-growing body of clinical evidence and health care algorithms to create software applications that drive high quality clinical decision making. Think Research's solutions are provided through a secure, multi-application platform that is used by clinicians and patients at the point of care, and is deployed in over 1,000 facilities in Canada, the US and the EU, with millions of patients being impacted annually.
SOURCE Think Research
Advancing research to reduce the cancer burden at the Canadian Cancer Research Conference
VANCOUVER, Nov. 6, 2017 /CNW/ - Researchers from across Canada will share discoveries at the 2017 Canadian Cancer Research Conference (CCRC), Canada's only national forum showcasing the entire spectrum of cancer research. The conference, hosted by the Canadian Cancer Research Alliance will be at the Vancouver Convention Centre from November 5–7. It is made possible by the supportership of several organizations, including cancer research funders and industry.
"Research is the only way through which the knowledge needed to decrease the death and suffering from cancer can be gained," said Dr. David Huntsman, Professor of Pathology and Laboratory Medicine and Obstetrics and Gynaecology at The University of British Columbia and staff pathologist at the BC Cancer Agency. "The CCRC provides an opportunity for cancer researchers to learn about novel ideas, tools and approaches, liaise with patients, inspire new investigators and trainees, and continue to make progress in our efforts to understand the inherent complexities of cancer."
Planning of the scientific program was led by three notable cancer scientists: Drs. Gerald Batist (Segal Cancer Centre and McGill University), Shoukat Dedhar (BC Cancer Agency and The University of British Columbia) and Christine Friedenreich (Alberta Health Services and The University of Calgary). This year's meeting will feature several important plenaries on topics as diverse as the burden of cancer from the epidemiological, economic, and psychosocial perspectives, the bold new world of cancer immunotherapies, and our growing understanding of the key metabolic changes at play in cancer cells. A slate of research leaders from Canada and abroad will be speaking at the 1,000-delegate conference. Nearly 600 posters will be presented over the three days.
In addition to the scientific program, the conference will host its inaugural Patient Involvement in Cancer Research Program, where patients and caregivers interested in advancing the cancer research agenda by incorporating the patient voice, will learn about the research process, their prospective roles in it, and interact with scientists in the spirit of mutual learning.
The "Celebration of Science" lecture will take place from 5:30 p.m. to 7:00 p.m. PT on Monday November 6. This public event will showcase the achievements of Dr. Connie Eaves, Distinguished Scientist at the Terry Fox Laboratory, BC Cancer Agency and Professor of Medical Genetics at The University of British Columbia. Dr. Eaves will share her personal journey as a cancer researcher, her own world class research in normal and cancer stem cell biology, and her observations on how our understanding of cancer has evolved over the past half century.
Access the scientific program here: http://conference.ccra-acrc.ca/.
Learn more about the Canadian Cancer Research Alliance (CCRA), visit: http://www.ccra-acrc.ca.
SOURCE Canadian Cancer Research Alliance
Online community brings innovation offering to more than 2 million Québec caregivers
WE THE CARING: Through social connection, Huddol.com empowers caregivers to create better care experiences and health outcomes for themselves and their loved ones
MONTREAL, Nov. 6, 2017 /CNW/ - At any given moment, more than 2 million Québec households are providing care to a family member or friend with a long-term health condition, disability or aging need.1 While many do not immediately identify themselves as a caregiver– with many seeing what they do as part and parcel for being a parent, sibling, child, spouse, friend or neighbour, the magnitude of the caregiving task cannot be ignored.
The reality is, many long-term caregivers face years of distress, disorientation and isolation as they work to map out a solution for their family member or loved one, often without knowing what questions to ask or which path will lead to the best outcomes.
Huddol.com – a digital innovation emerging from Québec, is turning those caregiving masses into a powerful social community that is crowdsourcing solutions to everyday care challenges. Huddol makes it easier than ever before for Canadians to navigate the long-term care of a loved one, while also taking care of their own needs.
"The suffering families face when they become caregivers has a way of turning them in on themselves; there's the stigma of the illness, the time it takes away from social relationships, and the sheer intensity and complexity of the task of having someone else's life in your hands," says Mark Stolow, CEO of Huddol. "Huddol is focused on reversing that trend and using the power of smart, caring connections to improve the health of caregivers and those in their care."
Innovative in its approach, Huddol empowers caregivers to create better care experiences and health outcomes for their loved ones by ensuring that they are treated as critical partners in care from the start. Using data inputs from each individual caregiver, Huddol smart matches members to a dedicated network of their peers, professionals and supportive resources. Time and time again research and real-world evidence emphasizes the importance of social connectivity in improving health outcomes. Through its collective insights and rich social networking, Huddol generates a powerful learning and sharing environment helping everyday people successfully navigate the care experience.
Québec singer-songwriter and La Voix contestant, Elie Haroun recounts his personal struggle and difficulty in witnessing the challenges his family faced when his father's cancer progressed into dementia in 2010.
"When my dad was dying, we were a team of two – me and my mom, but we were so lost in the experience," says Haroun. "I felt like I was losing both parents – one who was dying and the other who was self-sacrificing to the point of compromising her own mental health and self-care. Looking back, it would have meant everything to us to be able to tap into the collective trust of so many others – professionals, but also people like us who had been there or were going through the same experience."
With organizations such as Parkinson Québec, Alzheimer Society of Montreal and ALS Québec on board, and more than 150 professionals across the health and support service sector already as partners, Huddol provides caregivers with exclusive and easy-to-navigate access to an expansive integrated social and health eco-system that brings together all critical caregiver support touch points – private and public resources mixed within peer networks.
"Through their own determination and perseverance, many caregivers are trying to solve complex health riddles," says Monique Nadeau, Executive Director of L'Appui pour les proches aidants d'aînés. "Through the platform, Huddol fosters an online community that brings caregivers, organizations and professionals together. We believe that this initiative, which compliments existing services and aligns with our mission, will enable more caregivers to gain access to resources and supports."
Huddol's lead financial contributors include the Government of Canada's New Horizons for Seniors Program, Telus and L'Appui pour les proches aidants d'aînés. Huddol was developed by the seasoned team at The Caregiver Network – a group of health innovators, technologists, creatives, educators, researchers and clinicians with more than 15 years of experience working within the caregiver support space and innovating the future of caregiver support.
Caregivers across Québec can sign up for free access at huddol.com or download the Huddol iOS app.
About The Caregiver Network (TCN)
The Caregiver Network (TCN) is Canada's largest online learning network supporting family caregivers, their loved ones and the health care professionals who work on their behalf. TCN hosts free educational events in partnership with associations across the country. Events are led by experts who share up to date information and respond to questions from participants to help them better navigate the care journey. For more information on The Caregiver Network visit www.thecaregivernetwork.ca.
____________________________________
1 Statistics Canada. (2013). Portrait of caregivers, 2012. Catalogue no. 89-652 X- No.001. Ottawa, ON: Statistics Canada Social and Aboriginal Statistics Division, retrieved from http://www.statcan.gc.ca/pub/89-652-x/89-652-x2013001-eng.htm
SOURCE Huddol
The Ontario Produce Marketing Association Partners with Student Nutrition Ontario to Implement the "New Fast Food" Initiative in Over 1,000 Schools Across the Province
TORONTO, Nov. 6, 2017 /CNW/ - The Ontario Produce Marketing Association (OPMA) has partnered with Student Nutrition Ontario (SNO) to help kids make healthier choices when it comes to food. The "New Fast Food" campaign, consists of four, unique vibrant posters that have been distributed to 1,200 primary grade, middle and high schools, in the province.
"The concept of the program is quite simple," states Virginia Zimm, President of the OPMA. "No matter what age the students are, this series of posters will create a response in the student population that may provoke better, healthier fast food choices like fresh produce! What could be faster than an apple?"
The first of their kind to go into any school in Ontario, the pictorial representations are meant to transcend any language or cultural boundaries that are sure to exist in our diverse society. Children of all ages and backgrounds will understand the message the posters are conveying and hopefully encourage dialogue with friends, teachers, parents and caregivers.
In addition to the physical posters, the OPMA in partnership with SNO, is launching a social media campaign to support this initiative. Each poster has the OPMA "producemadesimple.ca" website across the bottom. Students and parents are encouraged to visit this website and follow them on Instagram, Facebook and Twitter. These tools will provide interested parties with access to content concerning everything from how to select, handle, store, and prepare fresh fruits and vegetables. They will also have access to hundreds of 'simple' recipes they can try for themselves or with the assistance of their parent or guardian, depending on their age.
Let's inspire our youth to make better food choices for their continued good health!
About Student Nutrition Ontario: At Student Nutrition Ontario, we stand for community, collaboration, and student success. We empower communities to address local needs and ensure that every student has access to nutritious foods, learns to make healthy choices, and is able to succeed. To support our partners, we bring knowledge, guidance, experience and passion. We offer a unified voice with the power to influence, build capacity, and ensure every student is well nourished.
About Ontario Produce Marketing Association: The Ontario Produce Marketing Association is a member-funded, not-for-profit organization whose primary objective is to increase the consumption of fresh fruits and vegetables in Ontario.
SOURCE Ontario Produce Marketing Association
Ebates Holiday Survey: Apple’s iPhone X vs. Samsung Galaxy
Survey reveals that age matters when it comes to smartphone preferences
SAN FRANCISCO—November 8, 2017—In the battle between Apple’s iPhone and Samsung’s Galaxy, American teens and adults are split on which one they want as a holiday gift. Over a third (35 percent) of teens said they either want the pricey iPhone X or the Apple iPhone 8. Only 20 percent of adults want the iPhone X, with a slightly higher percentage (22 percent) wanting the iPhone 8. Overall, adults said the Samsung Galaxy S8 is the smartphone they’d prefer to unwrap (38 percent). The national survey was fielded among 1,034 adults and 507 teens by Propeller Insights on behalf of Ebates, a leader and pioneer in cash back shopping and subsidiary of the global internet services company Rakuten.
| Which smartphone would you most like to receive? |
Percent of adults who said this |
Percent of teens who said this |
| 1. Samsung Galaxy S8 |
38 percent |
28 percent |
| 2. Apple iPhone 8 Plus |
23 percent |
25 percent |
| 3. Apple iPhone 8 |
22 percent |
35 percent |
| 4. Apple iPhone X |
20 percent |
35 percent |
The Trendy Teen Gifts Are…
If you’re planning to buy your teen a pair of Beats wireless earbuds, as 28 percent of American parents are, you’re in luck—at 44 percent, it’s the number one trendy teen gift that teens most want to receive.
Teen boys also want a drone (47 percent), while girls want a Polaroid camera (52 percent). Other trendy teen gifts for both teen boys and girls are:
| Which of the following popular gifts is at the top of your wish list? |
Percent of teen boys who said this |
Percent of teen girls who said this |
| 5. Beats wireless earbuds |
38 percent |
49 percent |
| 6. Nintendo Switch |
38 percent |
30 percent |
| 7. Bose portable speaker |
28 percent |
25 percent |
| 8. Amazon Echo |
26 percent |
24 percent |
However, while 27 percent of parents think that fidget spinners are still a hot gift for teens, only 15 percent of teens put this on their wish lists.
Non-Electronic Gifts Are Popular, Too
When asked which non-electronic gift Americans most want to receive, the most popular choice was a gift card—for both adults and teens (both 77 percent). Adults also put clothing (49 percent), shoes (35 percent) and accessories (31 percent) at the top of their list. Teens agreed and also listed clothing (63 percent), shoes (55 percent) and accessories (44 percent) as their most desired gifts.
Getaways were also a popular choice for Americans (32 percent). In fact, almost three- quarters—72 percent—would forego a gift in exchange for a holiday getaway.
When it comes to which type of gift card Americans would most like to receive, money in the form of a Visa, Amex or Mastercard (72 percent) was the top choice. This is followed by a gift card to a restaurant (59 percent), a retailer (51 percent) or for travel.
And men should take note: one-third of women also selected a gift card for a spa treatment (35 percent).
One-in-five Americans also plan to spend between $250-$500 on holiday gifts this year, and almost as many will spend more than $1,000 (18 percent) or $750-$1,000 (17 percent).
"Our holiday survey found that, when it comes to holiday gifts, you can’t go wrong with a gift card—although a laptop or earbuds are also safe choices,” said Amit Patel, CEO of Ebates. “At Ebates, we want to make it as easy as possible to purchase all your gifts and save money while doing it. From gift cards to fidget spinners, our retailers have you covered.”
From now through Christmas, shoppers can receive up to double cash back on select retailers via Ebates. For more details on specific retailers participating in the special offer, visit Ebates.com. Shoppers can also download the free Ebates.com app at Google Play and the AppStore.
About Ebates
Ebates rewards members with cash back on purchases while also providing access to thousands of coupons, discounts, promotions and special deals, including free shipping. Free membership allows consumers to shop online at over 2,000 of their favorite top-name retailers while earning a percentage of every purchase they make. To date, Ebates members have earned nearly $1 billion, paid out quarterly in the form of a “Big Fat Check” or via PayPal. Because shopping with Ebates is hassle-free—no rebate forms to fill out and no points or miles to redeem—the site supports a strong community of savvy shoppers across the country and around the world. Founded in 1998 and acquired by Rakuten, Inc. in 2014, Ebates is headquartered in San Francisco.