Kent Imaging's Breakthrough Medical Imaging Device Licensed with Health Canada
CALGARY, Nov. 7, 2017 /CNW/ - Kent Imaging Inc., a leading innovator in multispectral oxygenation imaging, announced today that their handheld KD203 is a licensed medical device with Health Canada. Kent's device is also cleared with the U.S. Food and Drug Administration (FDA) and the company is ISO13485 certified.
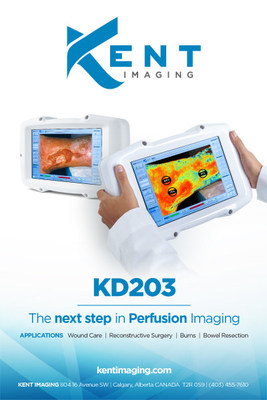
Kent's handheld KD203 measures and displays quantified tissue oxygen saturation levels in superficial tissue. Tissue oxygen saturation measurements provide critical information to help physicians determine at risk tissue and guide treatments to optimize patient outcomes. Kent's easy to use device is available for sale in the United States and Canada with utility in plastic surgery, colorectal surgery, trauma, wound care, limb salvage, podiatry, burns and cardiac specialties.
"The Kent Imaging camera is unique in its ability to assess oxygenation in tissue using spectroscopy," said Dr. Earl Campbell MD, FRCSC, practicing surgeon and a member of the Alberta Medical Association and the Canadian Society of Plastic Surgeons. "Plastic surgeons in Calgary, Alberta are already exploring its intra-operative uses when performing breast reconstruction procedures."
"We are happy to announce our licence with Health Canada," said Pierre Lemire, CEO of Kent Imaging. "This, along with its FDA clearance, has made our handheld device, the KD203, readily available to the physicians and surgeons who require the valuable insight it provides."
Features of Kent's KD203:
- Instant and precise visualization of oxygen saturation
- Non-invasive with no injectable dyes
- Ability to image the actual wound bed and surrounding tissue
- Oxygenation measurement of surgical sites or reconstructive flaps
- A means to track patient progress and treatment effectiveness
- Ease-of-use with minimal training required
- Accurate, unlimited imaging
- Lightweight and completely portable handheld device
About Kent Imaging Inc.
Kent Imaging Inc., a leading innovator in multispectral oxygenation imaging, designs, manufactures, and markets imaging technology to improve health care. The team at Kent Imaging is committed to improving patient outcomes by delivering innovative and practical solutions for the healthcare industry. Kent Imaging holds multiple patents in medical technology and is currently commercializing the breakthrough multispectral imaging solutions that assesses tissue oxygen saturation. The company is headquartered in Calgary, Alberta, Canada, and has locations in Chicago, Los Angeles, Salt Lake City and Seattle.
Kent's KD203 is U.S. Food and Drug Administration 510(k) cleared, and Health Canada licensed. The device is available for sale in the United States and Canada. Improving on Kent's previous KC103, the handheld Kent KD203 is intended for use by healthcare professionals as a non-invasive tissue oxygenation measurement system that reports oxygen saturation (StO2), relative oxyhemoglobin level (HbO2), and relative deoxyhemoglobin (Hb) level in superficial tissue. Kent's multispectral imaging technology displays two-dimensional color-coded images of tissue oxygenation of the scanned surface and reports multispectral tissue oxygenation measurements for selected tissue regions. Tissue oxygen saturation measurements provide critical information to help physicians determine at risk tissue and guide treatments to optimize patient outcomes.

SOURCE Kent Imaging
