Abbott's FreeStyle Libre® 2 System Now Reimbursed in Quebec
- Quebec joins Ontario and Prince Edward Island in offering access to both FreeStyle Libre and FreeStyle Libre 2 flash glucose monitoring technology
- The addition of the FreeStyle Libre 2 system enables access for Quebec residents aged four and over who manage their diabetes (type 1 or type 2) with multiple daily injections of insulin
- With a simple scan, the innovative FreeStyle Libre 2 delivers accurate, real-time glucose readings and alerts users when their glucose is too high or too low, without the need for routine finger pricks∞
MISSISSAUGA, ON, Aug. 16, 2022 /CNW/ - Abbott today announced that its FreeStyle Libre 2 flash glucose monitoring system* is now reimbursed under the Régie de l'assurance maladie du Quebec (RAMQ) for Quebec residents aged four and over who manage their diabetes (type 1 or type 2) with multiple daily injections of insulin~. Quebec was one of the first Canadian provinces to reimburse flash glucose monitoring technology and with the addition of FreeStyle Libre 2, families with children who are living with diabetes will have the opportunity to benefit from this important diabetes management tool.
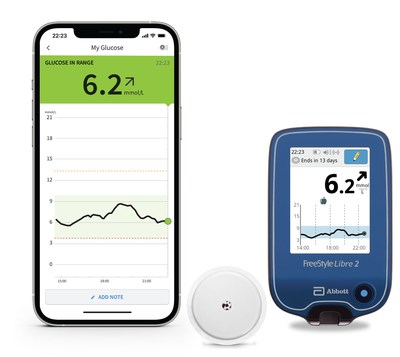
The FreeStyle Libre 2 system represents a significant advancement in diabetes management for Canadians. The sensor, which is the size of a Canadian Toonie, is worn on the back of the upper arm for up to 14 days. It measures glucose every minute to help people living with diabetes and their healthcare team make informed treatment decisions. With a one-second scan of a smartphone using the FreeStyle Libre 2 app,α users can see their glucose reading, trend arrow and eight-hour history. Using Bluetooth technology, the FreeStyle Libre 2 system can automaticallyπ alert users when their glucose is too high or low without needing to scan the sensor. Users can also share data with their family members via the LibreLinkUp mobile app.ύ
"The best healthcare solution is the one that helps the most people, which is why we designed our FreeStyle Libre portfolio with access and affordability in mind from the very beginning," said Philippe Busque, general manager of Abbott's diabetes care business in Canada. "Reimbursement of the FreeStyle Libre 2 system in Quebec is an important step toward helping more people with diabetes get access and we continue to work with all provincial governments to improve access for all people with diabetes across Canada."
Abbott's FreeStyle Libre 2 system has outstanding accuracy1. No routine confirmatory finger pricks are required for treatment decisions, even when glucose is low, falling or rapidly changing. It has impactful real-world2 and clinical research,3,4,5,6 showing how the FreeStyle Libre system is associated with better glucose control7, decreased time in hypoglycemia3,4,5,6 and hyperglycemia3,4 more time in optimal glucose range,7 and improved HbA1C.2 When compared to traditional blood glucose monitoring, using a flash glucose monitor can improve the ability of an individual living with type 1 or type 2 diabetes to monitor and manage their chronic condition along with their healthcare team, which is key to better diabetes management and health outcomes.
"This is great news for those living with diabetes in Quebec," said Sylvie Lauzon, president and CEO, Diabète Québec. "Providing access to flash glucose monitoring can help improve a person living with diabetes' ability to self-monitor, which is key to better diabetes management, and which can really help improve the quality of life of people living with diabetes."
As the #1 sensor-based glucose monitoring system used worldwide,∂ Abbott's FreeStyle Libre portfolio has now changed the lives of more than 4 million people across more than 60 countries.≠
Quebec announced public reimbursement of the FreeStyle Libre for the province in 2019. Public coverage is currently available for the FreeStyle Libre system in Ontario,£ Quebec,¤ Prince Edward Islandµ and Yukon# for those that meet the criteria. Public coverage is currently available for the FreeStyle Libre 2 system in Ontario,££ Yukon,Ø Manitoba,ΩSaskatchewan,± Prince Edward Islandµ and now Quebec¤¤, for those that meet the criteria. The FreeStyle Libre and FreeStyle Libre 2 flash glucose monitoring systems are reimbursed by most private payers in Canada,√ and are available to be electronically adjudicated at pharmacies with a prescription.
The FreeStyle Libre 2 flash glucose monitoring system is indicated for measuring interstitial fluid glucose levels in people aged 4 years and older with diabetes mellitus. Always read and follow the label/insert.
To learn more about the FreeStyle Libre 2 flash glucose monitoring system, visit www.MyFreeStyle.ca.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com, on LinkedIn at www.linkedin.com/company/abbott-/, on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews
ADC-59271v1.0 August 2022
DISCLAIMERS
| ∞ Finger pricks are required if your glucose readings and alarms do not match symptoms or expectations. | |
| ~ Those over the age of 4 and under the age of 18 who have type 1 diabetes are eligible for reimbursement. The system is also reimbursed for those 18 and older who manage their diabetes with insulin with three or more injections per day and who have had frequent episodes of hypoglycemia in the past year despite adhering to a blood glucose management plan. | |
| α The FreeStyle Libre 2 app and the FreeStyle Libre 2 reader have similar but not identical features. Finger pricks are required if readings do not match symptoms or expectations. The FreeStyle Libre 2 sensor communicates with the FreeStyle Libre 2 reader that started it or the FreeStyle Libre 2 app that started it. The FreeStyle Libre 2 app is only compatible with certain mobile devices and operating systems. Please check the website for more information about device compatibility before using the app. | |
| π Notifications will only be received when alarms are turned on and the sensor is within 20 feet of the reading device. You must have override do not disturb settings enabled to receive alarms and alerts on your smartphone | |
| ύ The LibreLinkUp app is only compatible with certain mobile devices and operating systems. Please check www.librelinkup.com for more information about device compatibility before using the app. Use of LibreLinkUp and FreeStyle Libre 2 app requires registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: home users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app | |
| ∂Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal-use sensor-based glucose monitoring systems. | |
| ≠ Data on file. Abbott Diabetes Care. | |
| √Individual private drug plans can vary. Please check with your plan administrator and/or your insurance company. | |
| * The FreeStyle Libre 2 flash glucose monitoring system is indicated for measuring interstitial fluid glucose levels in people aged 4 years and older with diabetes mellitus. Always read and follow the label/insert. | |
| £ Ontario: For people [over 18 years of age] managing diabetes with insulin. FreeStyle Libre Executive Officer Notice, Ontario Ministry of Health. September 12, 2019. | |
| ¤ Quebec: For people who meet the following two criteria: over 18 years of age on intensive insulin therapy (insulin pump treatment or ≥ 3 insulin injections per day) and with frequent episodes hypoglycemia in the last year. List of Medications, July 10, 2019. Régie de l'assurance maladie du Québec (RAMQ). FreeStyle Libre Changement dans les critères de remboursement. April 29, 2020. | |
| µ Prince Edward Island: For people on multiple daily injections of insulin (three or more) or using an insulin pump. Government of Prince Edward Island. June 1, 2022. | |
| # Yukon: For people with type 1 diabetes. Permanent funding for glucose monitors available to adult Yukoners, Government of Yukon, September 14, 2020. | |
| ££ Ontario: For people [over four years of age] managing diabetes with insulin. Ontario Drug Benefit Formulary Edition 43 (gov.on.ca). December 17, 2021. | |
| Ø Yukon: For people with type 1 diabetes. Government of Yukon Memo, April 22, 2021. | |
| Ω Manitoba: For people 25 years of age or under with type 1 diabetes who meet criteria. Manitoba Drug Benefits and Interchangeability Formulary Bulletin #113. Manitoba Health and Seniors Care. September 28, 2021. | |
| ± Saskatchewan: For children and youth under the age of 18 who meet criteria. Saskatchewan Formulary Bulletin #201. Saskatchewan Heath. June 1, 2021. | |
| ¤¤ * Quebec: For self-monitoring of blood glucose in diabetic persons aged four and over. Upon the initiation of treatment, the person: under age 18 must be suffering from type 1 diabetes; age 18 and over must meet the following two criteria: intensive insulin therapy (treatment by insulin pump or ≥ 3 insulin injections per day); and frequent episodes of hypoglycemia in the last year, despite compliance with a glycemic management plan. The initial request is authorized for a period of six months to assess the ability of patients to use FreeStyle Libre 2™ and wear the sensor. Requests for continuation of treatment are authorized for a maximum period of 12 months if the person shows optimal use of FreeStyle Libre 2™ or FreeStyle Libre™, i.e. at least 70% of the time. | |
| √ Individual private drug plans can vary. Please check with your plan administrator and/or your insurance company. |
REFERENCES
| _________________________________________ |
| 1 Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol 2020:1-8. |
| 2Evans M., Diabetes Ther. (2022): http://doi.org/10.1007/s13300-022-01253-9 |
| 3 Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016 Nov 5;388(10057):2254-2263. doi: https://doi.org/10.1016/s0140-6736(16)31535-5. |
| 4 Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krӧger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018 Mar;61(3):539-550. doi: https://doi.org/10.1007/s00125-017-4527-5. |
| 5 Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial. Diabetes Ther. 2017 Feb;8(1):55-73. doi: https://doi.org/10.1007/s13300-016-0223-6. |
| 6 Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of Flash Glucose-Sensing Technology for 12 months as a Replacement for Blood Glucose Monitoring in Insulin-treated Type 2 Diabetes. Diabetes Ther. 2017 Jun;8(3):573-586. doi: https://doi.org/10.1007/s13300-017-0255-6. |
| 7 Calliari et al. Real world flash glucose monitoring in Brazil: can sensors make a difference in diabetes management in developing countries? Diabetol Metab Syndr (2020) 12:3, https://doi.org/10.1186/s13098-019-0513-z |



SOURCE Abbott Diabetes Canada
