AbbVie reaches an agreement with the pan-Canadian Pharmaceutical Alliance (pCPA) for SKYRIZI™ (risankizumab) for the Treatment of Moderate to Severe Plaque Psoriasis
- Psoriasis is a chronic condition affecting 125 million people worldwide and many patients despite treatment still do not reach their goals or lose treatment response over time. 1-3
- SKYRIZI™ is a novel, humanized immunoglobulin monoclonal antibody designed to selectively inhibit IL-23 by binding to its p19 subunit.4
- On April 17, 2019, SKYRIZI™ received a NOC from Health Canada for the treatment of moderate to severe plaque psoriasis in patients who are candidates for systemic therapy or phototherapy, based on results from clinical studies showing significant improvement in levels of skin clearance after just 16 weeks and at 52 weeks with every 3-month dosing in more than 2000 adult patients. 4
- In May 2019, SKYRIZI™ received positive reimbursement recommendations from the Canadian Agency for Drugs and Technologies in Health (CADTH) and the Institut national d'excellence en santé et en services sociaux (INESSS).
- SKYRIZI™ is the first IL-23 inhibitor to arrive to a positive conclusion with the pCPA.
MONTREAL, Dec. 6, 2019 /CNW/ - AbbVie (NYSE: ABBV), a global, research and development-based biopharmaceutical company, announced an agreement was reached with the pan-Canadian Pharmaceutical Alliance (pCPA) regarding SKYRIZI™ (risankizumab) for the treatment of moderate to severe plaque psoriasis in patients who are candidates for systemic therapy or phototherapy.
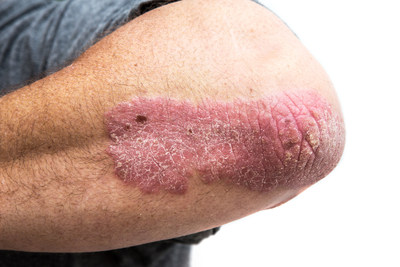
"Although the introduction of biologics has improved treatment outcomes, many patients continue to live with their needs unmet. Patients strive for therapies that provide predictable and durable full skin clearance with convenient dosing. Ultimately, they want a therapy that improves their quality of life,'' Dr. Kim Papp, Probity Medical Research Inc. ''It is great news to know that more Canadians will have access to a new treatment option."
SKYRIZI™ received Health Canada approval in April 2019 based on results from four pivotal Phase 3 studies, ultIMMa-1, ultIMMa-2, IMMvent and IMMhance evaluating more than 2,000 patients with moderate to severe plaque psoriasis.4 SKYRIZI™ is part of a collaboration between Boehringer Ingelheim and AbbVie, with AbbVie leading development and commercialization globally.
Canadians living with moderate to severe plaque psoriasis were well represented in all four of the pivotal clinical trials leading to Health Canada's approval, showing the Canadian leadership in this clinical development program. In clinical studies, SKYRIZI™ significantly improved levels of skin clearance after just 16 weeks and maintained clearance at one year (52 weeks).4
"We are committed to continuing to improve the lives of those living with psoriasis where there is still much to be done," explains Stéphane Lassignardie, Vice-President and General Manager, AbbVie Canada. "We are extremely proud that SKYRIZI™ is the first IL-23 inhibitor to reach an agreement with the pCPA. This is a great step forward for Canadians to obtain access to this innovative therapy."
About AbbVie Care
The AbbVie Care program is designed to provide a wide range of customized services including reimbursement and financial support, pharmacy services, lab work reminders and coordination, personalized education and ongoing disease management support throughout the treatment journey. For more information, consult www.abbviecare.ca.
About AbbVie
AbbVie is a global, research and development-based biopharmaceutical company committed to developing innovative advanced therapies for some of the world's most complex and critical conditions. The company's mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.ca and www.abbvie.com. Follow @abbvieCanada and @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
| References: | |
| 1. | International Federation of Psoriasis Associations. Available at: https://ifpa-pso.com/wp-content/uploads/2017/01/Brochure-Psoriasis-is-a-serious-disease-deserving-global-attention.pdf. Accessed March 22, 2019. |
| 2. | Mroweitz, U., et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011 Jan; 303(1): 1–10. |
| 3. | Levin, et al. Biologic fatigue in psoriasis. J Dermatolog Treat. 2014 Feb;25(1):78-82. doi: 10.3109/09546634.2013.826341. |
| 4. | SKYRIZI™ (risankizumab) [Canadian Product Monograph]. AbbVie Corporation, 2019. |
SOURCE AbbVie
